Addressing Challenges in Access to Oncology Medicines Analytical Report
Challenges in access to oncology medicines: Policies and practices across the OECD and the EU
From OECD report:
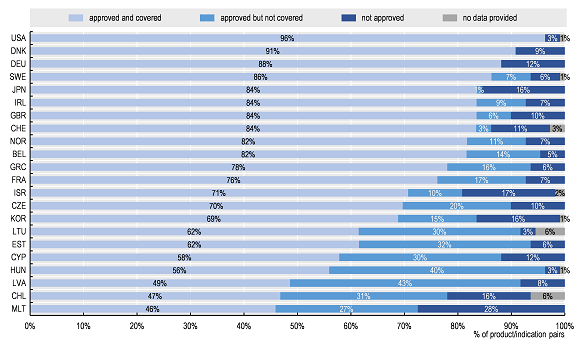
The data clearly show that access to oncology medicines is unequal across the OECD and EU. Of the 109 product/indication pairs in the sample, the United States had the largest percentage of product/indications approved and covered (by Medicare), followed by Denmark and Germany (96%, 91%, and 88%, respectively). Chile and Malta had the lowest percentage approved and covered (47% and 46% respectively). While access was more homogeneous for a subset of essential medicines (i.e. those included in the WHO 21st Model List of Essential Medicines [WHO-EML]), some of the 31 newer product/indication pairs were not yet approved (or launched) in some countries.
Percentage of total sample of 109 product/indication pairs by approval and coverage status across OECD/EU countries
Note: Data for some countries were not included as many data were missing.Source: Authors based on 2019 OECD survey on challenges in access to oncology medicines.