The Populist Temptation: Economic Grievance and Political Reaction in the Modern Era
31 de gener 2021
30 de gener 2021
29 de gener 2021
A plea for public patents on COVID prevention and treatment
Funding of Pharmaceutical Innovation During and After the COVID-19 Pandemic
Extensive public investments also are being made in therapeutics. The 2 most prominent monoclonal antibodies (by Regeneron and Lilly) have come to market with substantial governmental support for product commercialization. Both products derive from therapeutic research platforms established with governmental support before the COVID-19 pandemic, but product commercialization and manufacturing received major additional investments in 2020. Separately, the National Institutes of Health (NIH) Rapid Acceleration of Diagnostics program has committed $1.5 billion to supporting development of diagnostic tests related to COVID-19. The specifics of the federal contracts largely remain confidential.
Why do they remain confidential?
The lesson of the COVID-19 experience is that, when innovation in the life sciences is imperative, the traditional reliance on pharmaceutical industry prices and profits is jettisoned in favor of governmental grants and procurement. Sustained public funding for product development and commercialization will permit the sustained financing of innovation, a renewed attention to major public health needs, and the global position of the US pharmaceutical industry.
If there is public funding, why there aren't public patents?
28 de gener 2021
Technology assessment effectiveness
And the answer is YES.
This study suggests that medicine utilisation does respond to the positive recommendations of HTA bodies.However, if HTA capacity is organised primarily regionally, considerable effort may be required in coordination, to ensure consistent and rigorous assessments and adequate implementation of HTA findings.
27 de gener 2021
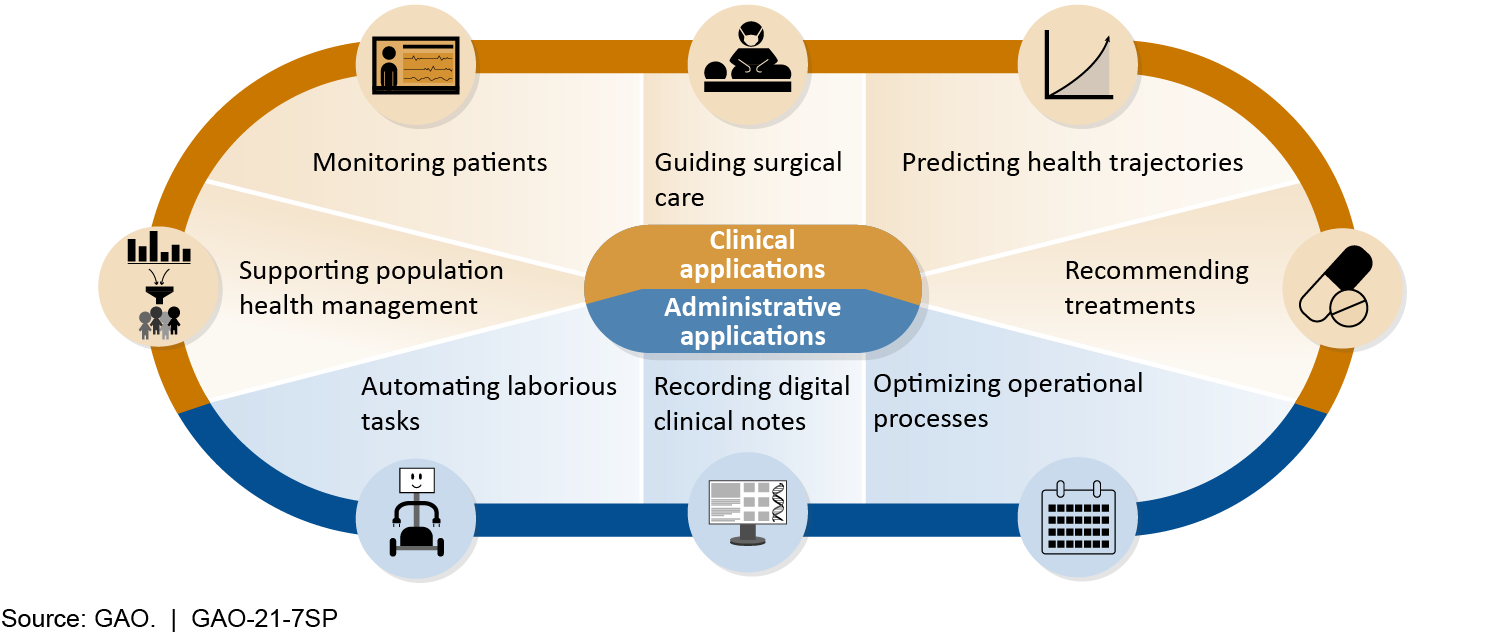
AI in Health Care
This report is being jointly published by the Government Accountability Office (GAO) and the National Academy of Medicine (NAM). Part One of this joint publication is the full presentation of GAO’s Technology Assessment: Artificial Intelligence in Health Care: Benefits and Challenges of Technologies to Augment Patient Care. Part Two is the full presentation of NAM’s Special Publication: Advancing Artificial Intelligence in Health Settings Outside the Hospital and Clinic.
Policy Options to Address Challenges or Enhance Benefits of AI to Augment Patient Care
| Policy Option | Opportunities | Considerations |
Collaboration (report p. 32)
|
|
|
Data Access (report p. 33)
|
|
|
Best Practices (report p. 34)
|
|
|
Interdisciplinary Education (report p. 35)
|
|
|
Oversight Clarity (report p. 36)
|
|
|
Status quo (report p. 37) Policymakers could maintain the status quo (i.e., allow current efforts to proceed without intervention). |
|
|
Source: GAO.
26 de gener 2021
Health systems during the pandemic
Health system responses to COVID-19
The Health System Response Monitor (HSRM) platform, a major initiative led by the European Observatory on Health Systems and Policies, the WHO Regional Office for Europe and the European Commission has published an issue that explains what's goin on in health services in the current pandemic, under the following issues:
- Covid-19 and health systems resilience
- Preventing transmission
- Ensuring sufficient workforce capacity
- Providing health services effectively
- Paying for services
- Governance
25 de gener 2021
CRISPR therapeutic success
Treatment by CRISPR-Cas9 Gene Editing — A Proof of Principle
CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia
Transfusion-dependent β-thalassemia (TDT) and sickle cell disease (SCD) are severe monogenic diseases with severe and potentially life-threatening manifestations. BCL11A is a transcription factor that represses γ-globin expression and fetal hemoglobin in erythroid cells. We performed electroporation of CD34+ hematopoietic stem and progenitor cells obtained from healthy donors, with CRISPR-Cas9 targeting the BCL11A erythroid-specific enhancer. Approximately 80% of the alleles at this locus were modified, with no evidence of off-target editing. After undergoing myeloablation, two patients — one with TDT and the other with SCD — received autologous CD34+ cells edited with CRISPR-Cas9 targeting the same BCL11A enhancer. More than a year later, both patients had high levels of allelic editing in bone marrow and blood, increases in fetal hemoglobin that were distributed pancellularly, transfusion independence, and (in the patient with SCD) elimination of vaso-occlusive episodes.
A pivotal moment. Great.
24 de gener 2021
Prioritizing vaccines
Who Goes First? Government Leaders and Prioritization of SARS-CoV-2 Vaccines
In US,
The vice president, Senate majority leader, House speaker, surgeon general, and members of Congress were among the first people vaccinated against Covid-19 in the United States. The president-elect, incoming first lady, vice president-elect, and her husband were also vaccinated in the first phase. Yet prioritization frameworks created by expert panels and adopted by states do not grant government leaders any special status, and giving them priority raises important questions of fairness and transparency. As vaccination proceeds, will other political or institutional leaders, or even celebrities and elite athletes, decide that prioritization plans don’t apply to them?
In another country, military personnel stole 300 vaccines that were for health personnel and administered themselves. I suggest you may guess which is this country....
23 de gener 2021
Genethics in practice
The ethics of genomic medicine: redefining values and norms in the UK and France
This paper presents a joint position of the UK-France Genomics and Ethics Network (UK-FR GENE), which has been set up to reflect on the ethical and social issues arising from the integration of genomics into routine clinical care in the UK and France. In 2018, the two countries announced enhanced cooperation between their national strategies, Genomics England and Plan France Médecine Génomique 2025, which offers a unique opportunity to study the impact of genomic medicine and relevant policies in different national contexts. The paper provides first insights into the two national strategies and the norms, values and principles at stake in each country. It discusses the impact of genomic medicine on established relationships and existing regulations, and examines its effects on solidarity and trust in public healthcare systems.
A must read for neighbour countries that have forgotten their homework.
22 de gener 2021
Mazzucato as a supplier of a flattering narrative for politicians (2)
Mission Economy. A Moonshot Guide to Changing Capitalism
My former post on a recent book by Mazzucato was based on a comment by McCloskey. Now, she has published a new one, and the best comment has been made by John Kay, clear message, I don't have anything to add.
Ever since 1969, people have asked themselves why if humans can land on the moon, can’t they solve pressing problems here on Earth, such as poverty, dementia and climate change. Mariana Mazzucato offers an answer: if only governments would apply the mission-driven methods of the Apollo project, they could.
Mission Economy, the new book from the high-profile economist noted for her advocacy of a more active state, contains many screenshots of the whiteboards beloved of brainstorming meetings, each with an ambitious goal at the top: secure the future of mobility, clean oceans, defeat cancer; below is a jumble of boxes and circles linked by multidirectional arrows.
We need a “solutions based economy”, driven and co-ordinated by more powerful governments engaged in every stage of the process of innovation.
But Apollo was a success because the objective was specific and limited; the basic science was well understood, even if many subsidiary technological developments were needed to make the mission feasible; and the political commitment to the project was sufficiently strong to make budget overruns almost irrelevant. Centrally directed missions have sometimes succeeded when these conditions are in place; Apollo was a response to the Soviet Union’s pioneering launch of a human into space, and the greatest achievement of the USSR was the mobilisation of resources to defeat Nazi Germany.
Nixon’s war on cancer, explicitly modelled on the Apollo programme, was a failure because cancer is not a single illness and too little was then — or now — understood about the science of cell mutation. Mao’s Great Leap Forward, a vain bid to create an industrial society within five years, proved to be one of the greatest economic and humanitarian disasters in human history. At least 30m people died.
Democratic societies have more checks and balances to protect them from visionary leaders driven by missions and enthused by moonshots, but the characteristics which made the Great Leap Forward a catastrophe are nevertheless still evident in attenuated version.
With political direction of innovation we regularly encounter grandiosity of ambition and scale; the belief that strength of commitment overcomes practical problems; an absence of honest feedback; the suppression of sceptical comment and marginalisation of sceptical commentators. All these were seen in Britain’s experience with Concorde, the Channel Tunnel and the AGR nuclear reactor programme, some of the worst commercial projects in history. More recently, there is the £12bn wasted on the NHS computerisation programme — a project that Mazzucato mentions, though only to blame private contractors for their failure to deliver on the political imperative.
On a smaller scale, Britain has suffered in the last year from the delays resulting from Public Health England’s insistence on central control of the coronavirus testing programme and the predictable fiasco of the attempt to sideline the expertise of Apple and Google in order to develop a uniquely advanced NHS test and trace app. And in September there was prime minister Boris Johnson’s “operation moonshot”, designed to control the coronavirus by testing 10m people daily in early 2021.
In contrast to these failures, the rapid development of vaccines is, at least provisionally, a success story. That development is not the product of visionary central direction but is the result of a competitive process with many different teams around the world attempting to be among the first across the finishing line.
Their work has drawn on a combination of existing academic science with the expertise in development and testing and the manufacturing and logistics capabilities of the global pharmaceutical industry. The role of government, appropriately, has primarily been in funding basic research and assuring that there will be a rewarding market for successful products.
Mazzucato lists “twenty things we wouldn’t have without space travel”. Athletic shoes, CAT scanners, home insulation, baby formula, artificial limbs. Yes, really. But beyond the ridiculous headline, we see the reality of productive innovation: a decentralised process in which developers draw on and help create the collective intelligence that leads to constant incremental improvement in so many fields — including better running shoes.
When historians of technology review the past 50 years, they may conclude that Neil Armstrong exaggerated when he announced “one giant leap for mankind”. The “new frontier” of the late 1960s turned out to be, not space, but information technology. And the development of IT was characterised by a striking absence of centralised vision and direction.
No moonshots; but piecemeal innovation through disciplined pluralism in which temporary winners were almost always displaced as they failed to anticipate the next step of the journey. Do you remember Digital Equipment, Word Perfect, Wang Laboratories, CompuServe, Netscape, AOL, BlackBerry? Each once a leader, now forgotten. Even Apple suffered more than one near-death experience, Microsoft failed to anticipate mobile computing or the cloud, IBM was swept out of the industry it had created.
Mazzucato has correctly emphasised the contribution of state funded basic research to Silicon Valley, but thank goodness the development was in the hands of Steve Jobs, Travis Kalanick and Elon Musk rather than a committee in the department of commerce.
No one has, or could have, the knowledge of present or future required to create or implement successfully the strategies that Mazzucato recommends. Take her modern signature example — Germany’s Energiewende, or energy transition to renewables. You will not learn from Mission Economy that this highly political, much publicised and wildly expensive project has brought about significantly smaller reductions in carbon emissions than Britain’s quiet, economically and socially beneficial substitution of gas for coal.
The failure of the Energiewende illustrates the dangers of moonshots and the mission economy. As talk of a “Green New Deal” becomes more frequent on both sides of the Atlantic, the prospect of more large, costly and ineffectual visionary projects grows.
Politicians readily fall in love with such proposals, and Mazzucato is not shy in reminding us how anxious they are to engage with her in discussing them. But the vision that propelled China’s economic development was not Mao’s Great Leap Forward or Cultural Revolution, but Deng’s “it doesn’t matter whether a cat is black or white if it catches mice”. It is more rewarding and effective to build better mousetraps than to shoot for a mice-free world.
John Kay is an economist, author and fellow of St John’s College, Oxford
21 de gener 2021
The loss of professional autonomy and the hegemony of marketplace medicine
Marketplace medicine has achieved such a strong ideological grip on our national consciousness, especially within the ranks of the health professions. Vested interests have been very persuasive in their propaganda against systems in other nations. Canada’s universal national health insurance model is maligned continually as unworkable here in the United States, even though our own Medicare system borrowed both its name and some structure from the Canadian national system—just without becoming universal for everyone! Americans do not realize how much of their money is wasted in this corporate healthcare system on overly priced, tax-supported care, coupled with such climbing out-of pocket personal payments for their families for this corporate healthcare system.
20 de gener 2021
19 de gener 2021
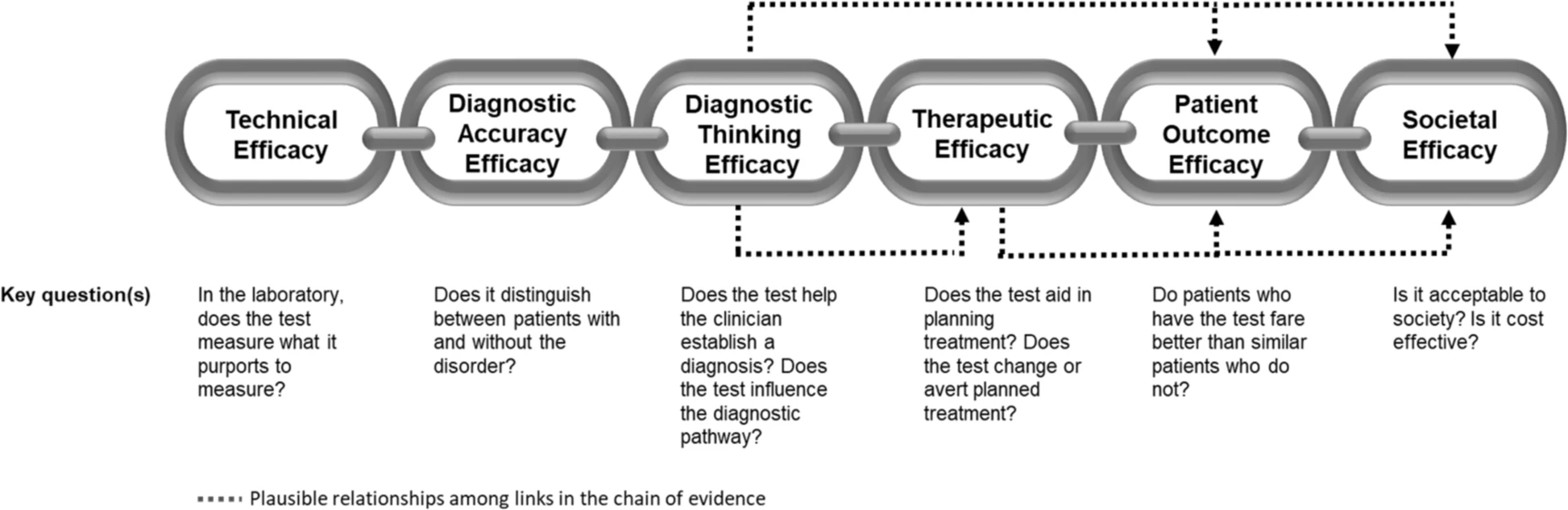
Clinical utility of genomic sequencing
Clinical utility of genomic sequencing: a measurement toolkit
From Genomic Medicine:
For a diagnostic test such as WGS (whole genome sequencing) to be accepted into practice, commissioned in a health system, or receive coverage and reimbursement through health insurance, evidence of clinical utility and cost-effectiveness is generally required. Unlike prospective clinical research where the ‘effectiveness’ of an intervention can be easily tied to a predefined health outcome, the concept of clinical utility in genetic medicine is rarely uniformly defined nor necessarily directly tied to a specific health outcome. As such, generating and evaluating evidence of clinical utility is complex. The challenge in defining clinical utility today is compounded by the extraordinary heterogeneity of rare diseases, as well as the polygenic nature of more common conditions for which WGS is expected to be relevant. In this paper, we aim to extend earlier conceptualizations of clinical utility as applied to the diagnostic use of WGS and suggest that this framework not only be used as a tool for evidence review
The application of this model to WGS includes six levels of efficacy: technical efficacy, diagnostic accuracy efficacy, diagnostic thinking efficacy, therapeutic efficacy, patient outcome efficacy, and societal efficacy (Table 1, Fig. 1). The model is hierarchical; achieving a given level of efficacy is often but not always contingent upon a demonstration of efficacy at the preceding level. As described in Fig. 1, levels 1–3 are necessarily contingent but beyond level 3, a genetic test can achieve therapeutic, patient outcome, and/or societal impact in ways that are contingent upon one another or independent of one another. We retain the levels of technical and diagnostic accuracy efficacy (i.e., levels 1 and 2) as essential starting points in our guiding framework as they are fundamental precursors to achieving clinical utility. However, since these laboratory-based components of efficacy are well-debated and described in the WGS literature and in recent guidelines published by members of our group27, we focus here on four levels of the efficacy model (i.e., levels 3–6) that align most directly with a broad definition of clinical utility and extend beyond laboratory-based components of efficacy. In emphasizing these four levels of efficacy as components of clinical utility, our intent is to encourage the use of a broad set of health and non-health-related indicators of value to bolster the state of evidence in this area, rather than to convey that all aspects of clinical utility need to be achieved for WGS adoption and reimbursement.
18 de gener 2021
CRISPR 2020, a breakthrough year
2020 Was the Turning Point for CRISPR
At an Oregon hospital in March, a patient with a type of inherited blindness became the first to receive a gene-editing injection directly into their eye. It was the first time CRISPR was used in an attempt to edit a gene inside someone’s body. A second person this year also received the experimental treatment, which is designed to snip out a genetic mutation responsible for their severe visual impairment.
Despite its versatility, CRISPR is still error-prone. For the past few years, scientists have been working on more precise versions of CRISPR that are potentially safer than the original. This year, they made notable progress in advancing these new versions to human patients.
17 de gener 2021
16 de gener 2021
15 de gener 2021
Precision medicine
Precision Medicine for Investigators, Practitioners and Providers
Many topics under the same umbrella:
Table of Contents
Introduction
2. Role of genomics in precision medicine
3. High throughput omics in the precision medicine ecosystem
4. Infant gut microbiome
5. Paraprebiotics
6. Fecal transplantation in autoimmune disease
7. Drug pharmacomicrobiomics
8. CRISPR technology for genome editing
9. Engineering microbial living therapeutics
10. Organ on a chip
11. Multicellular in-vitro organ systems
12. The role of biobanks in biomarker development
13. Translational interest of immune profiling
14. Organoid pharmacotyping
15. Large datasets for genomic investigation
16. Modern applications of neurogenetics
17. Genomic profiling in cancer
18. Genomics in pediatrics
19. Genomics of gastric cancer
20. Genomics of prostate cancer
21. MicroRNAs and inflammation markers in obesity
22. MiRNA sequencing for myocardial infarction screening
23. Cell free DNA in hepatocellular carcinoma
24. Non coding RNA in cancer
25. Germline variants and childhood cancer
26. Pharmacogenomics in cancer
27. Proteomic biomarkers in vireoretinal disease
28. Proteomics in respiratory diseases
29. Cardiovascular proteomics
30. Host genetics, microbiome, and inflammatory bowel disease
31. Sampling, Analyzing, and Integrating Microbiome ‘omics Data in a Translational Clinical Setting
32. Omics and microbiome in sepsis
33. Molecular and omics methods for invasive candidiasis
34. Lipid metabolism in colorectal cancer
35. Salivary volatolome in breast cancer
36. immunodiagnosis in leprosy
37. decision support systems in breast cancer
38. Electronic medical records and diabetes phenotyping
39. Clinical signature of suicide risk
40. Machine learning and cluster analysis in critical care
41. Artificial intelligence in gastroenterology
42. Algorithms for epileptic seizure prediction
43. Precision medicine in ophthalmology
44. Phenotyping COPD
45. Lifestyle medicine
46. Precision medicine for a healthier world
47. Aging and clustering of functional brain networks
48. Nutrigenetics
49. Genome editing in reproductive medicine
50. MRI guided prostate biopsy
51. Precision Nutrition
52. Theranostics in precision oncology
53. Precision medicine in daily practice
54. Imaging in precision medicine
55. Organoid for drug screening
56. Printing of personalized medication using binder jetting 3D printer
57. 3 D printing in orthopedic trauma
58. Consumer genetic testing tools in depression
59. The future of wearables
60. Tumor heterogeneity and drug development
61. Smartphone based clinical diagnosis
62. Smartphone biosensing for point of care use
63. Data security and patient protection
64. Blockchain solutions for healthcare
65. Ethical questions in gene therapy
66. Pitfalls of organ on a chip technologies
67. Regulatory issues of artificial intelligence in radiology
68. Academic industrial alliance
69. The future of precision medicine
70. Precision Medicine Glossary
71. Useful internet sites
14 de gener 2021
13 de gener 2021
12 de gener 2021
11 de gener 2021
10 de gener 2021
09 de gener 2021
Business rules
The Six New Rules of Business. Creating Real Value in a Changing World
1. Intangibles drive value, not the balance sheet: Reputation, trust, and other intangibles—not profit—drive business value and cannot be measured in traditional ways
2. Purpose over profits: Businesses serve many objectives beyond shareholder value. Purpose is more than a slogan; it is revealed through how the company operates and the decisions it makes. Taken seriously, the Purpose signals long term goals and helps the company prevail over short-term pressures.
3. Corporate Responsibility is more than your carbon footprint: Corporate responsibility is defined far outside the business gates—extending through the supply chain and ecosystem to an engaged public.
4. Employees are more than 'stakeholders' -- they are the business: Employees are corporate allies that hold businesses accountable, connect social and environmental issues to business priorities and give voice to risk and competitive advantage in a culture of growing inequality and social unrest.
5. Culture is King: Competition for talent and a focus on innovation and the human element take precedence over capital markets.
6. Co-create, don't compete: When the system is at risk, enlist business partners along the supply chain to raise the bar for the industry as a whole, and win.
08 de gener 2021
07 de gener 2021
Moments determinants en la política sanitària
Moments determinants en la política sanitària
My article in Valors magazine, January issue (in catalan):
La immediatesa i el dinamisme del món en que vivim ens porta massa sovint a considerar la política sanitària com el fruit de la decisió puntual, fruit d’una resposta a un problema o controvèrsia del moment. Pensem en polítics reactius i poc proactius, i massa sovint encertem. Ens cal una mirada més panoràmica per comprendre que darrera tota política sanitària cal trobar-hi uns valors socials que li donen fonament. És a dir, uns criteris sobre allò que considerem bo i just per a la societat. En ocasions es referiran als objectius o resultats finals i en d’altres als mitjans per assolir-los. Si bé la rellevància d’aquests criteris és determinant per als objectius, en relació als mitjans cal tenir present a més a més la seva efectivitat, i per tant un judici expert sobre allò que de veritat funciona.
L’objectiu final de tota política sanitària descansa sobre la millora de la salut poblacional però alhora va més enllà, alguns hi podríem afegir el benestar com a concepte. La resposta dels governs per assolir-ho és diversa segons les prioritats que mostren. L’any 2006 la Unió Europea va considerar que els sistemes de salut són un pilar de la protecció social, de la cohesió i de la justícia social, i va assenyalar que la universalitat, l’ accés a l’assistència de qualitat, l’ equitat i solidaritat són els valors europeus compartits[i]. És en aquest marc i en el seu desenvolupament, on es fonamenta la política sanitària.
L’adopció de decisions públiques en relació a aquests valors essencials requereix d’un consens polític. Pertoca al parlament i al govern assenyalar què signifiquen exactament aquests valors compartits (objectiu) i quines decisions cal adoptar per assolir-los (mitjans). L’existència d’un consens social sobre aquestes qüestions esdevé una peça clau de l’engranatge. Més enllà de l’acord polític parlamentari, cal que tots els actors que participen en el sistema de salut s’orientin cap a la mateixa direcció. I precisament un dels elements que es consideren factor d’èxit d’un sistema sanitari és el consens social. Ens cal doncs una política sanitària basada en el màxim consens possible per tal de tenir un sistema eficient i equitatiu que perduri.
Distingir aquells mitjans que poden ser efectius per assolir objectius d’eficiència i equitat d’aquells que no ho són, esdevé una prioritat. Disposar d’evidència per tal de contrastar aquelles decisions que produiran el millor resultat és crucial, però alhora complex. La complexitat prové de les singularitats i del context on es desenvolupa cada política. Allò que ha funcionat en un lloc i moment determinat, pot ser difícil de ser reproduït en un altre. Malgrat aquest atenuant, saber allò que funciona amb un criteri expert i objectiu ha d’ajudar a millorar les decisions. Molts informes d’experts sobre reforma sanitària han tractat d’adoptar aquesta perspectiva, si bé amb impacte força limitat. La falta d’aplicació del consell expert té a veure almenys amb les dificultats d’establir consensos amplis i la comprensió del procés polític.
La Comissió de Salut del Parlament de Catalunya va acordar el febrer de 2013 impulsar el treballs per assolir un acord per a la salut a Catalunya en el marc d’un model propi. Aquest acord es va traduir en el Pacte Nacional de Salut on es van definir les bases del sistema sanitari català, estables i consensuades per tots els agents implicats. Dins la comissió de treball hi havia els representants dels diversos grups parlamentaris i els agents que formen part del Consell Català de la Salut. Els treballs de la comissió es van desenvolupar en vuit grans àmbits temàtics. Es va prendre com a referent els 6 blocs inicials que segons l’Organització Mundial de la Salut han de constituir un sistema sanitari, i s’hi van afegir dos àmbits identificats com a claus pel sistema (la recerca i innovació, i el compromís ciutadà). Els àmbits van ser doncs: finançament i cobertura, professionals, prestacions i catàleg de serveis, model de serveis, avaluació i transparència, recerca i innovació, compromís ciutadà, i governança.
Entrar en el detall del contingut dels 83 acord va més enllà del que es pretén en aquest article, tant sols es farà una breu referència als 2 primers. En l’àmbit de finançament i cobertura s’assenyala amb claredat l’opció per un accés universal de la ciutadania al Sistema Nacional de Salut i alhora s’estableix un criteri de nivell de finançament públic en salut suficient i sostenible, que es relacioni amb el nivell de riquesa del país i que convergeixi amb la despesa de països amb producte interior brut per càpita equivalent i sistema sanitari similar.
En l’àmbit dels professionals es considera que la planificació de necessitats de professionals, les competències i capacitats acreditades han de ser objecte reconsideració atenent als canvis sociodemògràfics, econòmics i tecnològics. S’explicita l’èmfasi en el professionalisme com a criteri que guia la relació entre professionals amb la ciutadania, amb el sistema sanitari i els proveïdors, i la necessitat d’establir mecanismes per tal de fer efectiva la participació dels professionals en l’elaboració de polítiques i la gestió.
El procés per arribar a aquest conjunt d’acords va ser fruit d’una elevada participació. Diversos motius singulars van impedir que la totalitat dels representants confirmessin el seu acord al darrer moment. Va ser aprovat per tots els membres excepte partits a l’oposició i sindicats. Cal assenyalar que en l’elaboració del document hi van haver contribucions de tots, també d’entitats i grups polítics que van donar finalment el seu suport.
En el moment actual cal fer èmfasi novament en aquest consens que desitja la ciutadania, i les bases perquè això sigui possible hi són, només cal teixir-les acuradament i amb generositat. On són les dificultats per avançar doncs. Des del meu punt de vista, la polarització política fa perdre el nord sobre quins són els valors autèntics de la política sanitària. Quan es discuteix sobre els valors sovint hi ha més acord que conflicte. En canvi, la perspectiva partidista sobre els mitjans i decisions per assolir els objectius finals de millora de la salut poblacional fa confondre (intencionadament) el que es pretén amb la forma d’assolir-ho. I aleshores entra el tercer element, que és la necessitat d’eines i metodologies tècniques i expertes que en moltes ocasions obliguen a un esforç de comprensió major del que alguns acceptarien com a normal.
Novament el parlament ha demanat ara un nou pacte nacional de salut, sense fer cap referència al que ja es va fer. No som capaços ni d’aprendre del passat. El risc de confluir en els mateixos paranys és elevat i caldria un esforç seriós que permetés evitar-los. El moment en que es proposa tampoc ajuda, ens trobem a finals d’una legislatura i començament d’una altra. El context de la pandèmia ha fet veure a molts les mancances que ja coneixiem i havíem explicat anteriorment. Ara bé, insistir en un pacte sense cap capacitat de disposar de nous mitjans i recursos diferents als actuals pot comportar clarament un nova paràlisi de la política sanitària. Només un canvi substancial mitjançant una sobirania fiscal permetrà apalancar un nou sistema de salut d’acord amb els valors, expectatives i nivell de desenvolupament econòmic de Catalunya.
06 de gener 2021
05 de gener 2021
One Health
Introduction to One Health. An Interdisciplinary Approach to Planetary Health
Our decisions will come to affect the future in unknowable ways. Our past has shaped our today, and our current actions are setting the path forward for tomorrow. Some actions are permanent and irreversible. Irrespective of the path we, as a global community, choose, our actions will have lasting consequences for the health of our planet. Let us act for good. Let us act for One Health.