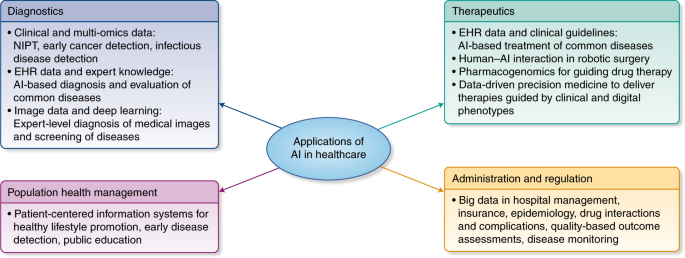
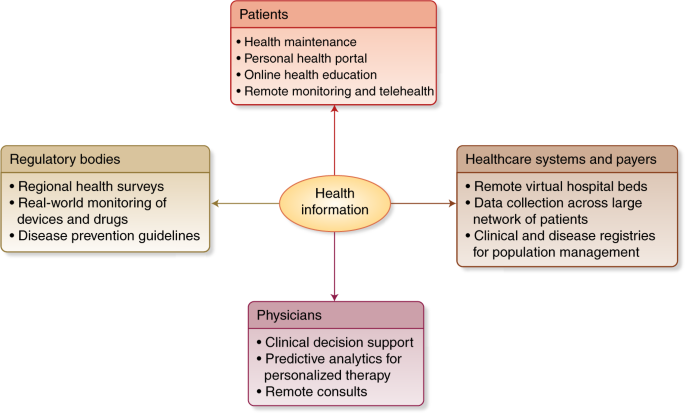
Currently medical diagnosis is driven by a standard way to proceed. We could say that the pattern of the decision flow has not changed for years.
A physician takes a history, performs an examination, and matches each patient to the traditional taxonomy of medical conditions. Symptoms, signs, family history, and laboratory reports are interpreted in light of clinical experience and scholarly interpretation of the medical literature.Data availability, and specifically genetic data could change completely diagnostic process.
Initiatives to develop genetic reference data at the population level could be grouped into 3 categories.First are well-known databases of genotype-phenotype relationshipsThe reference to these databases is crucial to understand what's going on in US medicine, and how european medicine stands behind.
as observed and submitted by researchers (eg, Online Mendelian Inheritance in Man, ClinVar, and the National Human Genome Research Institute’s Genome-Wide Association Study [GWAS] Catalog). Second are databases, such as the Genome Aggregation Database (gnomAD), the next iteration of the ExomeAggregation Consortium (ExAC) database, and the 1000 Genomes Project, that aggregate sequences
collected from other studies for secondary use. Third, patients and other study participants are invited to donate data to registries like GenomeConnect or enroll
in cohorts like the National Institutes of Health All of Us initiative, which is recruiting 1 million patients to contribute biological samples and EHR data for research.
JAMA article develops the concept of Clinical Information Commons:
There should be a new compact between patients and the health system, such that captured data and biospecimen by- products of the care deliverysystem should be aggregated and linked to build a clinical information commons (CIC) to aid diagnosisI agree. Saluscoop started as an alternative focused in this approach. As usual, the big question is: who is going to invest in a digital commons?. Unless governments take this initiative as a whole, the future of a data driven medicine is uncertain.

.jpg)