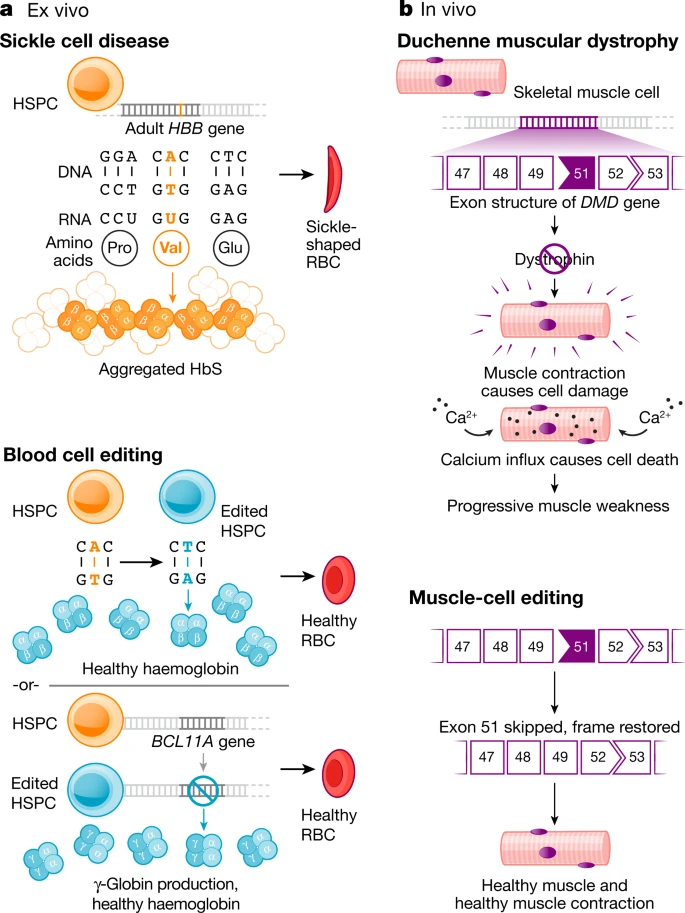
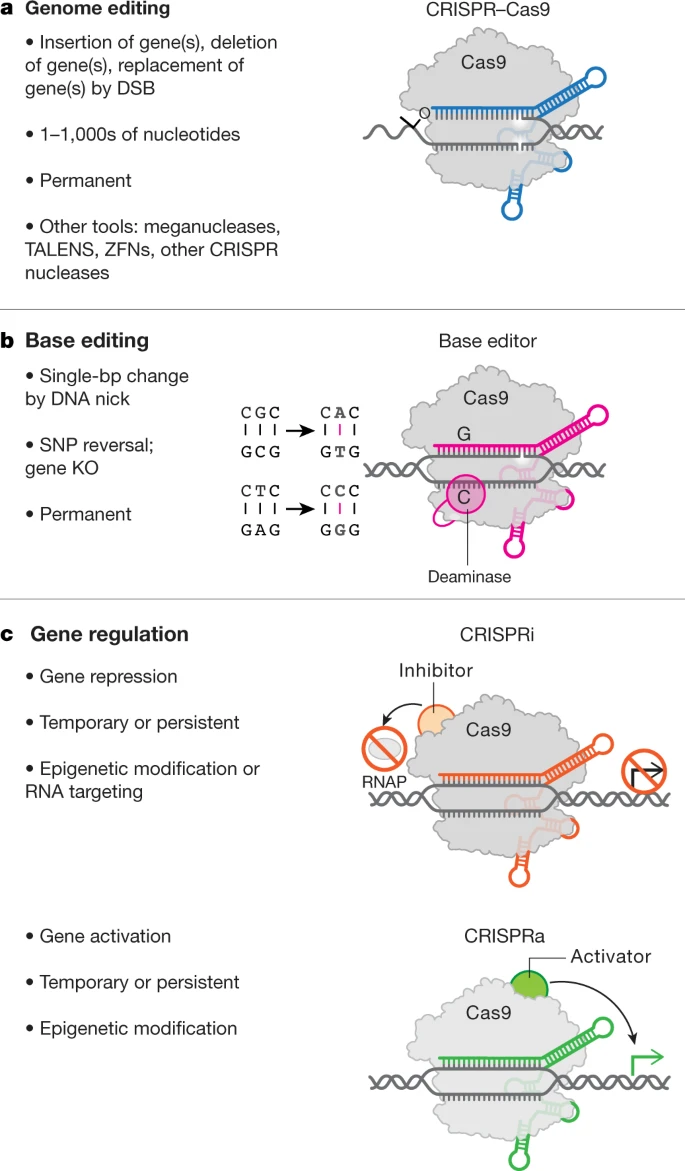
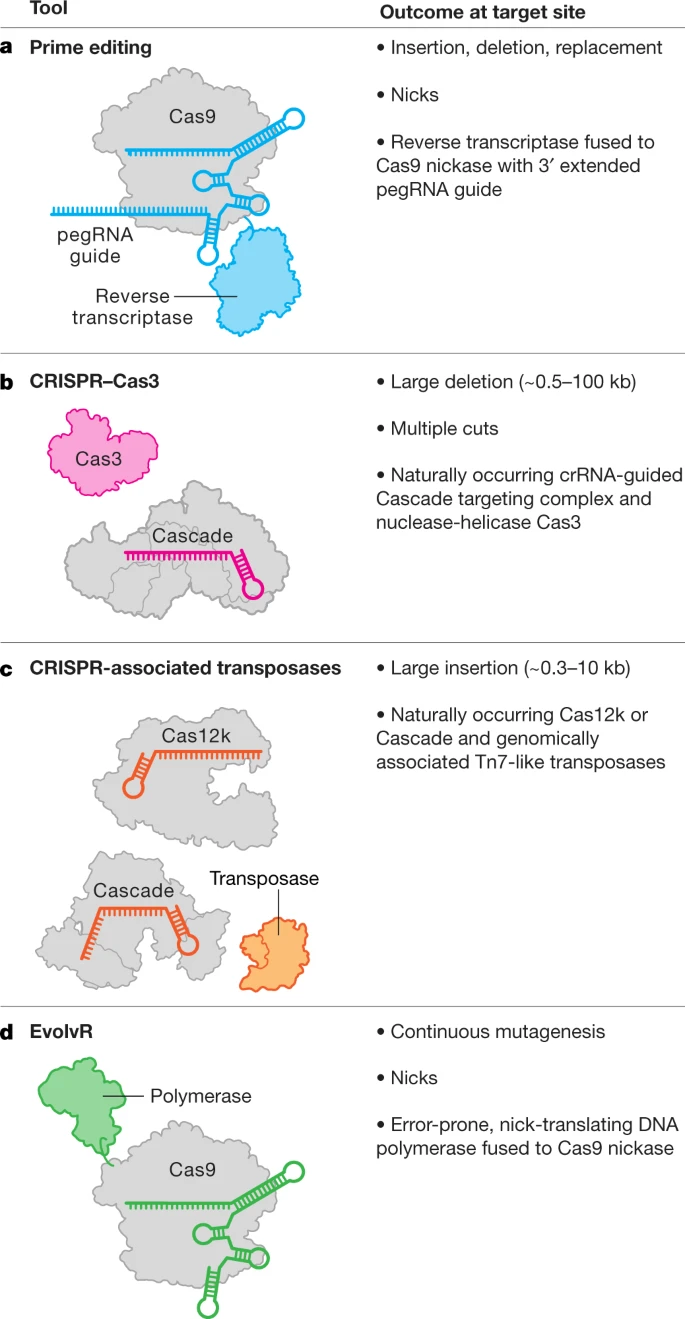
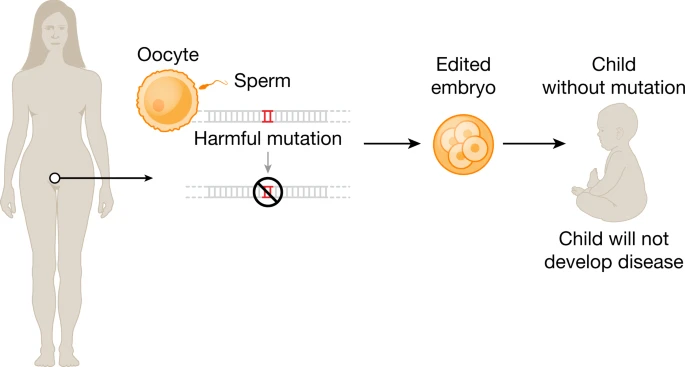
On Therapeutical Nihilism and effectiveness (Ch. 11), a philosophical view:
The confidence that a medical intervention is effective ought to be low, even when presented with evidence for that intervention’s effectiveness. How low? I do not think that there can be a precise or general answer. It is enough to say: lower, often much lower, than our confidence on average now appears to be. There is surprisingly little direct study of the confidence that physicians or patients or policy-makers have regarding the effectiveness of medical interventions. However, the confidence typically
placed in medical interventions can be gauged by the resources dedicated to developing, marketing, and consuming such interventions.
What explains the disparity between the confidence placed in medical interventions and the lower confidence that I have argued we ought to have? The ingenious techniques that companies use to market their products—paying celebrities to publicly praise their products, funding consumer advocacy groups, sponsoring medical conferences, influencing medical education, direct-to-consumer advertising—have been extensively discussed by others. The promise of scientific breakthroughs partly explains this disparity—scientists seeking support for their research programs, and companies building hype for their products, often make bold predictions about the promise of the experimental interventions they are researching, and this can sound convincing when it is put in the language of genomics, proteomics, precision medicine, personalized medicine, and evidence-based medicine. Unwarranted optimism may be based in part on a history of a few successful magic bullets, such as penicillin and insulin—magic bullet thinking gets inappropriately adopted in premature proclamations of game-changing medical interventions, which media outlets promulgate.
Medical nihilism is not the thesis that there are no effective medical interventions. Please do not confuse this. Medical nihilism is, rather, the thesis that there are fewer effective medical interventions than most people assume and that our confidence in medical interventions ought to be low, or at least much lower than is now the case.As I said, an unconventional and controversial view. We do need measures to assess facts and knowledge, philosophy is not enough. Anyway, I recommend its reading.