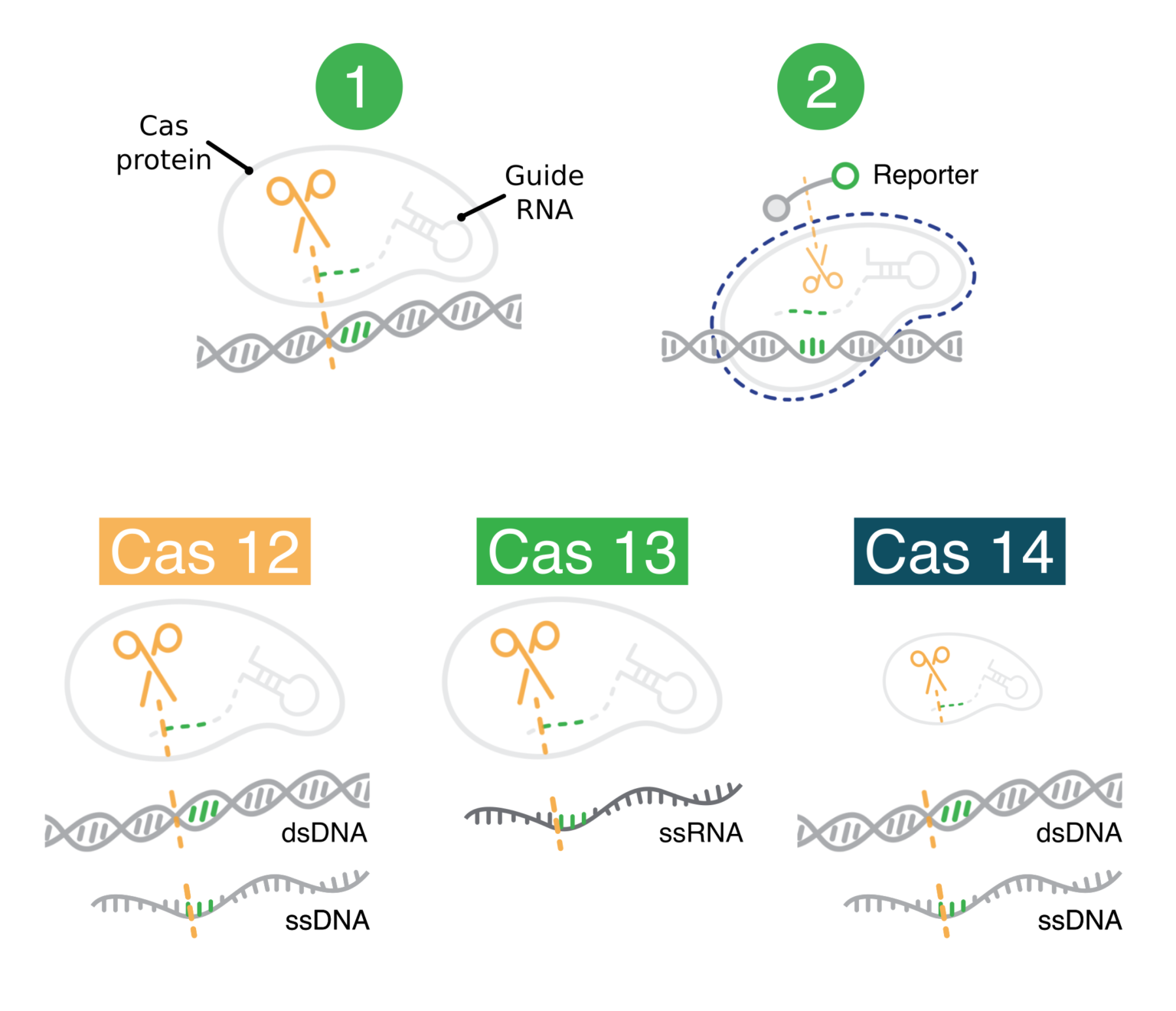
Assessing the Added Therapeutic Benefit of Ultra-Expensive Drugs
In US:
The number of ultra-expensive drugs and Medicare beneficiaries taking these drugs has grown significantly, resulting in a very high concentration of Medicare Part D spending on ultraexpensive drugs. Between 73% and 85% of these drugs assessed in France, Canada, or Germany received a low added therapeutic benefit rating. Policy reforms to address drug prices in the United States should consider developing an assessment framework for added therapeutic benefit to incentivize and reward the development of drugs that offer a significant clinical improvement over the current standard of care. In the interim, use of international assessments would be possible.
However, it may seem weird to our eyes, but:
Medicare Part D in particular has a problem with ultra-expensive drugs, since it pays nearly 80% of the cost of these drugs, and by law Medicare cannot directly negotiate the price for these drugs with the drug companies
Public funding without the possibility to set the contract for low marginal benefit drugs! The result:
Medicare Part D spending on brand-name drugs for these ultra-expensive drugs increased from 1.5% in 2012 to 19% in 2018
The answer is change the law and set benefits package according to added value.