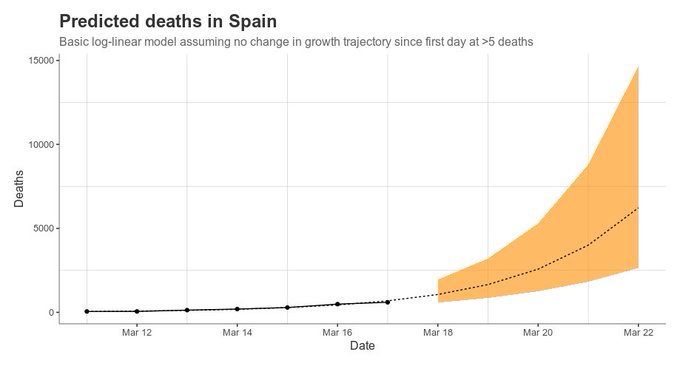
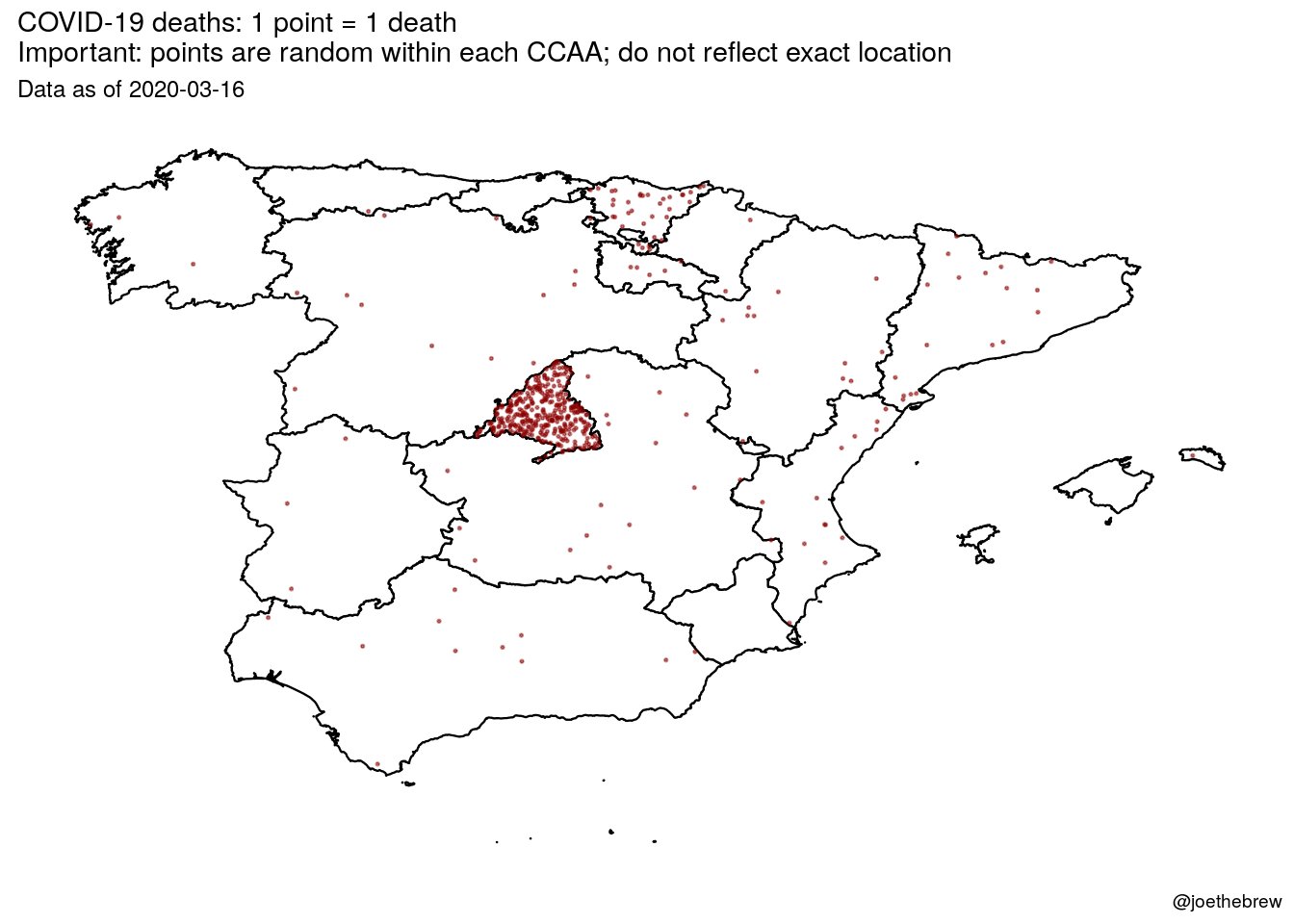
Some days ago I was explaining the rationale for coronavirus testing regarding clinical decision making. However, as we all know, individual behavior is also capable to produce health and disease contagion. Therefore, in case of coronavirus, behavioral externalities are crucial and nowadays we have denominated them "social distancing".
Having said that, there is an additional behavioral value from testing to take into account. If all individuals in a population have access to the test, maybe everybody is aware of social distancing than in a situation than only suspected cases receive the test. Behaviors may change, and quarantine strategies more successful. In such situation it is much more feasible to restrict mobility. Let's take for example what this article explains:This paper studies the effect of public policies to restrict migration by individuals suspected of carrying disease, when those individuals do not know for certain whether they have the disease but may have more information than the authorities about their probability of being carriers. It has long been known that migration affects the spread of
disease, and this influence has for centuries been used to justify placing restrictions on the movement of individuals suspected of carrying infections.
Epidemiological studies have addressed how individual behaviour, among other factors, affects the spread of infections. However, the study of how individual behaviour in turn
changes in response to the new incentives created by the occurrence of a disease is much less developed. The principal contribution of our paper is to bring the study of strategic behavior under uncertainty into the domain of epidemiology, and to analyze its impact, in interaction with public policies, on the overall impact of epidemic disease.
Migration as a form of preventive behaviour has received very little attention, although evidence has accumulated that migration behaviour and epidemics are intrinsically linked. Migration behaviour can respond very rapidly to changes in the health environment, in particular when it suddenly deteriorates through epidemics.
In our model we show that:
• First, when the disease is concentrated in one place (the epicentre of an epidemic for instance), a decision to migrate away from the epicentre brings a potentially infected individual in contact with more uninfected individuals than she would have met had she
remained where she was. Thus the typical migrant imposes a net negative externality as a result of her decision to migrate, and the marginal migrant (for whom, by definition, private benefits of migrating just equal the private costs of doing so) has a negative
impact on social welfare. Laissez faire will therefore lead to excessive migration. This provides a rationale for the frequent (and frequently justified) public policy response to epidemics, which is to attempt to restrict migration away from the epicentre by those who may be infected.
• Secondly, and less obviously, not all policies that aim to restrict migration in fact do so. In particular, we distinguish two effects of quarantine policies. The first is that they raise migration costs, which lowers migration. For example, mandatory health certificates or test results may be required by health authorities to leave the epicentre of the disease.We call this a “type 1” effect of quarantine measures. The second effect is that they impose a utility cost on individuals of remaining in the city where quarantine measures are effective, since they face a chance of being subjected to awkward and possibly
dangerous restrictions on their movements. We call this a “type 2” effect of quarantine measures. Such measures impose a welfare cost on those who suffer them, which tends to increase migration by those who are not currently subject to quarantine but fear they may become so if they remain where they are. Policies implemented without taking type 2 effects into account may therefore have results that are opposite from those intended.
• Thirdly, even policies that actually reduce migration may have an adverse impact on social welfare if they reduce migration “too much”, and specifically if they discourage those intra-marginal migrants whose private benefits from migration substantially
exceed their private costs of migration, by enough to outweigh the negative externality they impose on others. Overall disease prevalence may even increase if in the name of avoiding negative externalities the authorities discourage relatively low-risk individuals
from escaping the epicentre of the disease, thereby increasing the probability that they will catch the disease there from infected individuals.
When people have imperfect information about their own infection status, migration imposes net negative externalities by increasing the rate of exposure faced by the uninfected outside the epicentre of the epidemic. In and of itself, this our paper has highlighted the fact that although quarantine of individuals who have been identified as sick reduces (obviously) the propensity of these individuals to migrate and spread the disease, the threat of quarantine increases the propensity to migrate of other individuals who have not yet been fallen sick but who know themselves to be at risk.Quarantine measures have all these effects. However, if information about contagion is confirmed, then behaviors may change, and mandatory health certificates can be issued. The case of the italian village of Vò confirms that population screening has been successful in stopping the outbreak. This could have been done at the beginning if diagnostics kits had been available. Right now it seems an unfeasible strategy. We know now that there is a behavioral value of test information, beyond the clinical value. And in the case of coronavirus, confirmatory tests provide only partial information. In case of non infection, incubation period is uncertain, and some days after can be confirmed. Therefore, quarantine measures have to be mandatory and strict for the whole population and for specific areas.