Key principles:
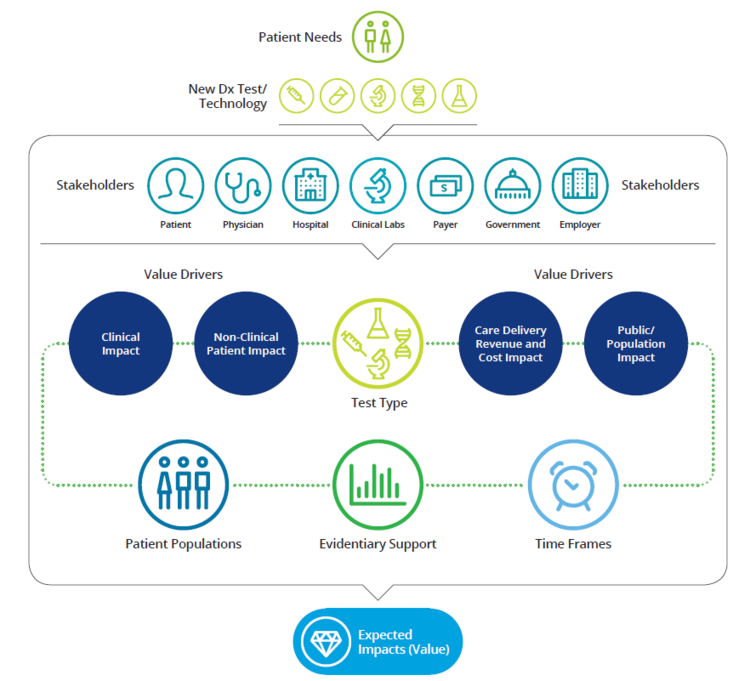
Value drivers can come from four major sources:
- Apply broad array of patient centric value drivers from various perspectives (Comprehensiveness principle)
- Utilize appropriate range of available evidence, reflecting test type and potential risks-benefits (Evidentiary principle)
- Consider reporting direct and indirect costs incurred and avoided over timeframe appropriate for the test (Cost principle)
- Account for immediate and longer-term test impact and patient benefits in representative patient populations (Specificity principle)
- Include quantified estimates as well as qualitative analyses as appropriate (Flexibility principle)
- Incorporate multiple stakeholders’ perspectives (Engagement principle)
- Disclose why the assessment was initiated, who was involved, its purpose, and the decision-making process (Transparency principle)
- Update assessments regularly to keep up with the rapid technological and clinical changes (Relevancy principles)
- Clinical impact: clinical utility and health outcomes associated with the diagnostic technology. The test needs to measure accurately and reliably the analyte/biomarker of interest (analytical validity); detect, predict the outcomes of interest in a patient population (clinical validity) and inform an appropriate clinical decision (clinical utility). Improved patient safety, tolerability, compliance and physical and psychological wellbeing shall be also taken into account.
- Non clinical patient impact breaks down to patient experience, and patient economics, such as proximity of test delivery, reduced follow-up visits, repeat procedures, improved care plan compliance and reduced burden on care givers.
- Care delivery revenue, and cost impact mostly refer to quality of care metrics and more efficient resource utilization (e.g. readmissions; follow-ups, length of stay, wait times)
- Public and population impact refer to macro implications mainly from population health, burden of disease, patient and caregiver productivity perspectives
AdvaMedDx’s Approach for Effective Value Assessment
Source: A Framework for Comprehensive Assessment of the Value of Diagnostic Tests, AdvaMed, 2017
And clinical impact depends on analytical validity, clinical validity, clinical utility, patient safety and patient response. If you have only one strategy for all the patients, like social distancing in the case of coronavirus, then the information post-test will not change the therapeutic strategy. If the test tries to prevent contagion when social distancing can't be applied (health professionals, politicians, journalists, executives, essential services), than you have to test them if there are symptoms. If the test will add information to existing comorbidities to differentiate from other symptoms, then it makes sense. Therefore, this is the current situation in my country. Test, test, test when it adds value.