Reviewing our methods for health technology evaluation: consultation
08 de desembre 2020
07 de desembre 2020
Access to effective medicines (in oncology)
Addressing Challenges in Access to Oncology Medicines Analytical Report
Challenges in access to oncology medicines: Policies and practices across the OECD and the EU
From OECD report:
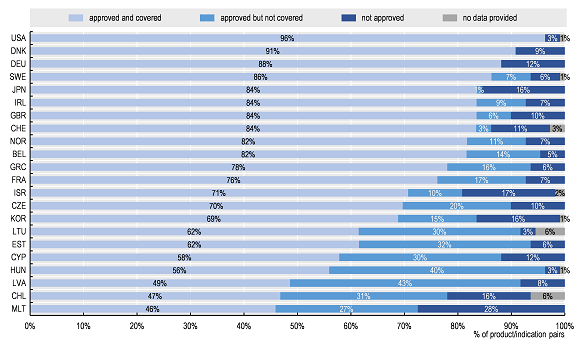
The data clearly show that access to oncology medicines is unequal across the OECD and EU. Of the 109 product/indication pairs in the sample, the United States had the largest percentage of product/indications approved and covered (by Medicare), followed by Denmark and Germany (96%, 91%, and 88%, respectively). Chile and Malta had the lowest percentage approved and covered (47% and 46% respectively). While access was more homogeneous for a subset of essential medicines (i.e. those included in the WHO 21st Model List of Essential Medicines [WHO-EML]), some of the 31 newer product/indication pairs were not yet approved (or launched) in some countries.
Percentage of total sample of 109 product/indication pairs by approval and coverage status across OECD/EU countries
Note: Data for some countries were not included as many data were missing.Source: Authors based on 2019 OECD survey on challenges in access to oncology medicines.
06 de desembre 2020
Fueling digital health
Healthtech in the fast lane: What is fueling investor excitement?
From McKinsey brief:
As of 2019, digital health represented a global market of approximately $350 billion with many opportunities to compete across multiple subcategories; moreover, the markets for technologies in every value pool are expected to grow by at least 8 percent per annum (Exhibit 2). Understandably, the bulk of digital health players develop technologies that have a direct impact on patient care. About 49 percent of the digital health companies we studied fall into the care-delivery category (that is, offering more effective therapies, providing remote patient support, or supplying therapies to patients)—a $157 billion market (as of 2019) comprising 45 percent of the overall digital health market. Companies in this category either provide novel therapeutic solutions enabled by digital technologies—such as Livongo for diabetes—or use technology to broaden patient access to healthcare solutions, for example, telemedicine company Teladoc (offering remote patient support) or online pharmacy PillPack (supplying therapies to patients). Notably, every value pool in this category is expected to grow by at least 10 percent per annum through 2024.
Digital health’s platform wars are heating up. From Rock health
Few seats available
05 de desembre 2020
Long term care shame
The share of LTC spending in total health spending or as a share of GDP has gradually increased over the last 15 years in many OECD countries as demand for care grows with population ageing and the extension of publicly financed services.
In some countries, spending on LTC has not grown much faster than the economy as a whole. In Slovenia and Spain, for example, the shares of LTC relative to GDP in 2018 were only 0.1-0.2 percentage points above those in 2005, at 1.2% and 0.9% of GDP, respectively.
In Spain, the share of LTC spending has been stagnating since 2009, with LTC spending tracking GDP growth.
04 de desembre 2020
Risks and benefits of self-testing (2)
Direct to Consumer Testing: The Role of Laboratory Medicine
A specific issue on the topic has been released in Clinics in Laboratory Medicine. Inside the issue, you'll find this article: Direct-to-Consumer Tests on the Market Today: Identifying Valuable Tests from Those with Limited Utility 13. This is a key topic. It says:
Debate exists between the consumer and the health care provider when it comes to the value of direct-to-consumer (DTC) testing. At the heart of the issue is the observation that consumers are identifying value in DTC testing as evidenced by an expanding market, and health care providers are skeptical of their value from an analytical and clinical utility perspective. The aim of this article is to briefly review the subject of DTC testing with a specific focus on value from the perspective of the consumer and the health care provider.
Paul Strand at KBr Barcelona
03 de desembre 2020
Trust in professions
In the UK, nurses and doctors are the most trusted professions, with 93 per cent and 91 per cent of the public saying that they trust them to tell the truth. Advertising executives, politicians and journalists receive the lowest scores
02 de desembre 2020
Investing in pandemic preparedness
The Cost Effectiveness of Stockpiling Drugs, Vaccines and Other Health Resources for Pandemic Preparedness
A short review on the topic, many questions, few answers.
Health economics methods can be used to decide the optimal pandemic preparedness strategy based on cost effectiveness because different stockpiling of available measures can be implemented. The economic evaluation of pandemic preparedness strategies and pandemic preparedness measures is based on methods developed for health technology assessment. Nevertheless, this assessment differs from the traditional economic evaluations. The cost-effectiveness evaluation of a new drug compares healthcare costs and health effects for patients treated and not treated with the drug. The cost effectiveness of the drug will depend on the effectiveness of the drug in reducing clinical outcomes and healthcare costs. The drug will be used by the health system in patients with certainty. In contrast to this, the cost-effectiveness evaluation of pandemic preparedness measures and interventions is affected by several facts. First, pandemic preparedness measures are costly because they must be used to prevent and treat pandemic infections in a great number of persons. Second, investments in pandemic preparedness measures could be made many years before the emergence of the pandemic pathogen. Third, the health and economic benefits generated by pandemic preparedness measures will depend on the virulence and infectiousness of the pandemic pathogen. Fourth, pandemic preparedness measures can be associated with large costs and benefits outside the health system and great macroeconomics effects. There is a risk that an unknown pandemic agent will emerge and cause high morbidity and mortality, but we do not know when this will happen or how virulent and infectious a new pandemic agent will be. Although a new pandemic can be similar to previous pandemics, it can be also very different.
Ventilator support and intensive care for acute respiratory failure due to acute respiratory distress syndrome is a cost-effective intervention [8], but the cost effectiveness of stockpiling ventilators depends on the number of stockpiled ventilators and the severity of a future pandemic. The cost effectiveness of ventilator support and intensive care ranges from US$29,000 per QALY in low-risk patients (≥ 70% probability of surviving at least 2 months from the time of ventilator support) to US$110,000 per QALY in high-risk patients (prognostic estimate ≤ 50%) [7]. The question about the optimal number of stockpiling ventilators for pandemic preparedness depends on intervention costs and uncertainty about when the pandemic will happen and how virulent and infectious the pandemic pathogen will be.
Paul Strand at KBr
01 de desembre 2020
Risks and benefits of self-testing
Benefits and Risks of Direct-to-Consumer Testing
These are the pros and cons of two options for the same production process and its impact (a US view only applicable to certain conditions). (Professionalism vs. commercialism).
Conventional:
Self-testing:
And its impact:
Potential and Perceived Benefits of Direct-to-Consumer Testing
Potential Risks of Direct-to-Consumer (DTC) Testing
With DTC testing, consumers may not know the risks or what significance a result has in terms of current and future health. It is challenging for consumers to distinguish tests for health and wellness from entertainment and commercial marketing. This can leave consumers open to misdiagnosis and susceptible to unproven treatments and questionable claims for cancer and disease cures. DTC tests are both an opportunity for an individual to better participate in their health and in the management of chronic diseases, as long as consumers are aware of the risks for inappropriate utilization and inadvertent interpretation that can lead to avoidable follow-up, unnecessary procedures, and additional costs of care. DTC testing is an emerging field and an opportunity for laboratory experts to participate through providing professional advice, consultation on test limitations, interpretive services, and recommendation on optimal test utilization.
My view, now it's time to stop recreational testing and commercialism of lab testing. Later it will be too late.
30 de novembre 2020
How monopolies make our lives harder every day & destroy our future
Monopolies Suck. 7 Ways Big Corporations Rule Your Life and How to Take Back Control
7 Key messages from the book:
ONE: MONOPOLIES TAKE YOUR MONEY
TWO: MONOPOLIES GOUGE YOU WHEN YOU’RE SICK
THREE: MONOPOLIES LOWER YOUR PAY AND CRUSH THE AMERICAN DREAM
FOUR: MONOPOLIES SPY ON AND MANIPULATE YOU
FIVE: MONOPOLIES THREATEN DEMOCRACY AND YOUR FREEDOM
SIX: MONOPOLIES DESTROY OUR PLANET AND CONTROL YOUR FOOD
SEVEN: MONOPOLIES RAMP UP INEQUALITY
So what? You'll find the details on how to take back control in Chapter 8, a set of clear tools to fight the monopolistic pandemic.
29 de novembre 2020
On ecosystems
ECOSYSTEM EDGE.Sustaining Competitiveness in the Face of Disruption
Selected statements from the book:
Each of the four ways in which the development of an ecosystem can unlock new value—through new product bundles, new customer solutions, new platform economies, and spawning new industries—shares a common characteristic: they require a process by which new customer value is discovered. The new value is not just assembled or delivered from existing elements by following a predetermined blueprint; it has to be identified. Business ecosystems come into their own by facilitating the process of discovery.
Discovering new sources of value requires the three key capabilities that ecosystems excel at: a huge potential for rapid, joint learning and innovation; the ability to harness the capabilities of diverse players and channel them toward a common goal through the leadership of an enlightened company; and the flexibility for continuous reconfiguration in the face of an uncertain, fast-changing environment.
Given these demands, what can you do as an ecosystem leader to catalyze and promote the discovery of new value through your ecosystem strategy?
The rise of ecosystems will also rewrite the rules of competition and strategy as we have come to know them, encapsulated in the seminal work of Michael Porter. The classic cost-leadership strategy based on growing the volume of products and services a company produces to reap economies of scale that drive down costs below competitors, will be replaced. In a world of competing ecosystems, cost advantage will come from aggregating the scale of your entire partner network to spread the fixed costs of investments in everything from design and innovation through to production and distribution. Your ability to reap network economies will become decisive.
As competition between ecosystems grows, rewriting the rules of competition in the process, the demands on ecosystem leaders are only set to increase. The pressure to discover new sources of value for customers and to craft unique and attractive offerings will intensify. Partners will evaluate whether joining an ecosystem can deliver benefits, as well as how one ecosystem’s value proposition compares with that of another network. A potential ecosystem leader’s value proposition to partners will need to be top-notch. In the competition between ecosystems, the speed with which a leader kick-starts a virtuous spiral of cooperation and the rate at which it can scale its ecosystem are both critical. Those that get ahead because of network economies will surge forward in what could easily become a winner-take-all game. However, to sustain the ecosystem leader’s competitive position, the amount of learning and innovation generated by the ecosystem, and its ability to capture that learning, will be critical. Ecosystems that are less productive and efficient will be unable to compete. Mastering the strategies and capabilities we described earlier will become even more essential tomorrow than they are today.
This is the only book that finally provides some clear conceptualisation on ecosystems., as far as I know.
28 de novembre 2020
27 de novembre 2020
The impact of COVID-19 on life expectancy
Our estimates of a 0.9 year decline in annual life expectancy in Spain suggest that the COVID-19 pandemic has the potential to cause life expectancy stalls not seen for decades.
In the first half of 2020, Spain has been one of the most affected countries both in terms of directly related COVID-19 deaths [1], as well as in terms of total excess mortality [4]. We estimated the annual life expectancy drop between 2019 and the one year window that closes out on 5 July, 2020 to be 0.9 years (~11 months) in Spain. In contrast, the average annual increase in life expectancy in Spain increased on average two months per year from 2009 to 2019. Altogether, this suggests that life expectancy drop between observed and expected annual life expectancy in the recent one year window would be around or below one year.
Annual life expectancy at birth in 2019, 2020*a and differences between periods for Spain
26 de novembre 2020
Health for all (once again)
Achieving Health for All: Primary Health Care in Action
From chapter 2 of the book, on life expectancy and GDP (Preston curve)
The list of best-performing countries is still limited because it is often the case that a country looks like it is deriving a high health output from minimal economic growth simply because it has had a prolonged spell of economic stagnation, and health improvements are simply occurring as a result of secular trends set up by past introductions of public health technology. Thus, what often appear to be “top-performing” countries in climbing the Preston Curve frequently appear successful because of stagnant and barely positive economic growth. The project of empirically identifying exemplary countries achieving good health at low cost or “punching above their weight” needs to reexamine foundational assumptions about (1) whether countries are actually engaged in transforming economic growth into better health and (2) what counts as a valid comparison group for a given country at a given time depending on its starting LEB.
25 de novembre 2020
Cheap crime
Financial Penalties Imposed on Large Pharmaceutical Firms for Illegal Activities
From JAMA:
Among 26 firms in our sample, 22 (85%) had financial penalties for illegal activities. The combined dollar value of financial penalties totaled $33 billion for 2003 to 2016. Eleven firms with financial penalties exceeding $1 billion in inflation-adjusted dollars accounted for $28.8 billion (88%) of the total penalties (Table 1). The firms with the highest penalties as a percentage of revenues (ie, >1%) were Schering-Plough, GlaxoSmithKline, Allergan, and Wyeth; the number of penalties for these firms varied between 1 (Allergan) and 27 (GlaxoSmithKline). Four firms had financial penalties that totaled less than $80 million and no more than 2 penalty settlements (Actavis [Watson], Roche Group, Genzyme, and Perrigo). All but 1 firm (Perrigo) engaged in illegal activities associated with penalties for 4 or more years. An additional 4 firms received no financial penalties for illegal activities during this period. The most common types of illegal activity involving penalties (Table 2) were pricing violations, off-label marketing, and kickbacks. The firms with the greatest variety in the types of illegal activities involving penalties were GlaxoSmithKline, Bristol Myers Squibb, and Merck. Three firms (Actavis, Allergan, and Perrigo) had penalties limited to a single violation type.
That's it.