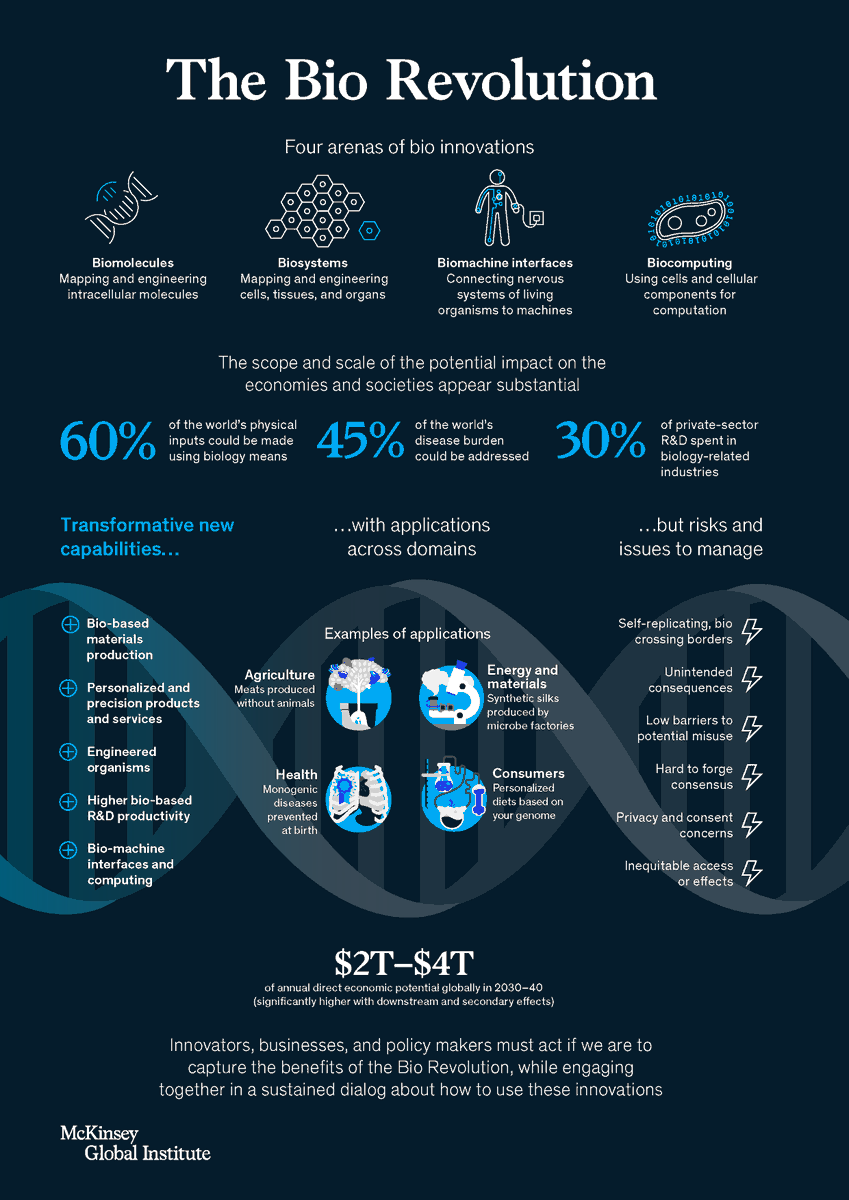
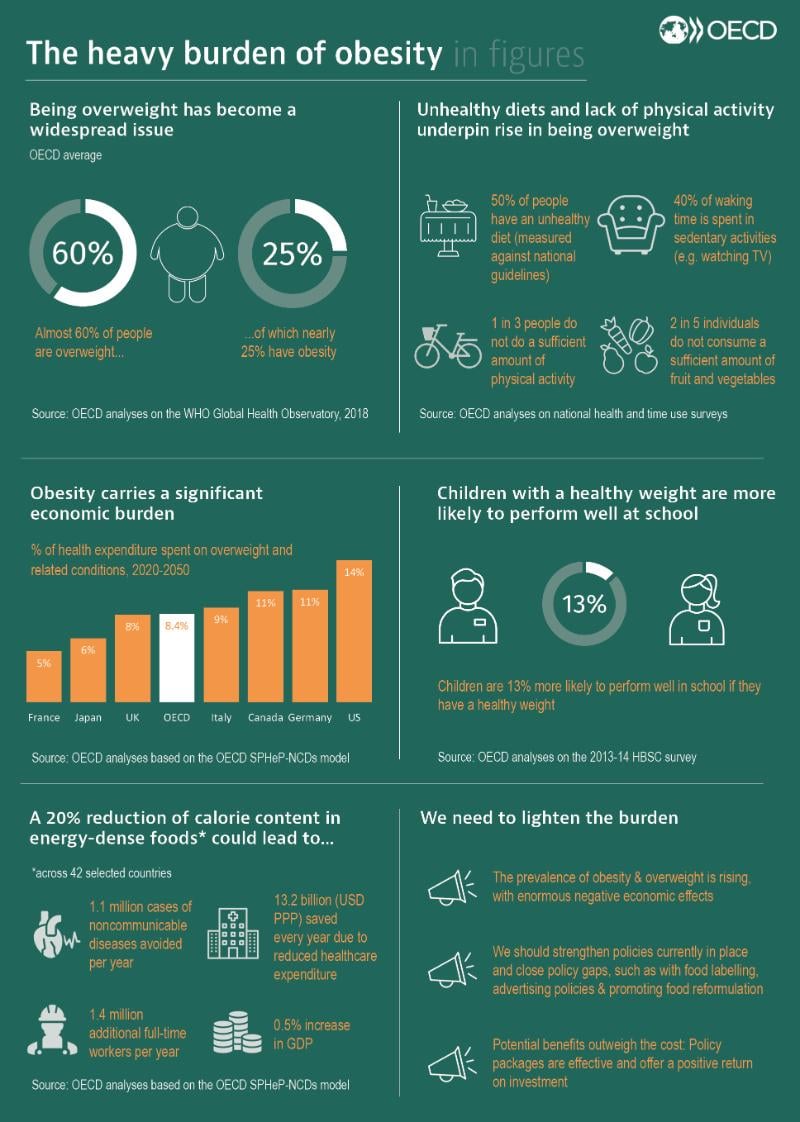
COVID-19 AND WORLD ORDER. THE FUTURE OF CONFLICT, COMPETITION, AND COOPERATION
Part I. Applied History and Future Scenarios
Chapter 1. Ends of Epidemics
Jeremy A. Greene and Dora Vargha
Chapter 2. The World after COVID: A Perspective from History
Margaret MacMillan
Chapter 3. Future Scenarios: "We are all failed states, now"
Philip Bobbitt
Part II. Global Public Health and Mitigation Strategies
Chapter 4. Make Pandemics Lose Their Power
Tom Inglesby
Chapter 5. Origins of the COVID-19 Pandemic and the Path Forward: A Global Public Health Policy Perspective
Lainie Rutkow
Chapter 6. Bioethics in a Post-COVID World: Time for Future-Facing Global Health Ethics
Jeffrey P. Kahn, Anna C. Mastroianni, and Sridhar Venkatapuram
Part III. Transnational Issues: Technology, Climate, and Food
Chapter 7. Global Climate and Energy Policy after the COVID-19 Pandemic: The Tug-of-War between Markets and Politics
Johannes Urpelainen
Chapter 8. No Food Security, No World Order
Jessica Fanzo
Chapter 9. Flat No Longer: Technology in the Post-COVID World
Christine Fox and Thayer Scott
Part IV. The Future of the Global Economy
Chapter 10. Models for a Post-COVID US Foreign Economic Policy
Benn Steil
Chapter 11. Prospects for the United States' Post-COVID-19 Policies: Strengthening the G20 Leaders Process
John Lipsky
Part V. Global Politics and Governance
Chapter 12. When the World Stumbled: COVID-19 and the Failure of the International System
Anne Applebaum
Chapter 13. Public Governance and Global Politics after COVID-19
Henry Farrell and Hahrie Han
Chapter 14. Take It Off-Site: World Order and International Institutions after COVID-19
Janice Gross Stein
Chapter 15. A "Good Enough" World Order: A Gardener's Manual
James B. Steinberg
Part VI. Grand Strategy and American Statecraft
Chapter 16. Maybe It Won't Be So Bad: A Modestly Optimistic Take on COVID and World Order
Hal Brands, Peter Feaver, and William Inboden
Chapter 17. COVID-19's Impact on Great-Power Competition
Thomas Wright
Chapter 18. Building a More Globalized Order
Kori Schake
Chapter 19. Could the Pandemic Reshape World Order, American Security, and National Defense?
Kathleen H. Hicks
Part VII. Sino-American Rivalry
Chapter 20. The United States, China, and the Great Values Game
Elizabeth Economy
Chapter 21. The US-China Relationship after Coronavirus: Clues from History
Graham Allison
Chapter 22. Building a New Technological Relationship and Rivalry: US-China Relations in the Aftermath of COVID
Eric Schmidt
Chapter 23. From COVID War to Cold War: The New Three-Body Problem
Niall Ferguson