20 de gener 2021
19 de gener 2021
Clinical utility of genomic sequencing
Clinical utility of genomic sequencing: a measurement toolkit
From Genomic Medicine:
For a diagnostic test such as WGS (whole genome sequencing) to be accepted into practice, commissioned in a health system, or receive coverage and reimbursement through health insurance, evidence of clinical utility and cost-effectiveness is generally required. Unlike prospective clinical research where the ‘effectiveness’ of an intervention can be easily tied to a predefined health outcome, the concept of clinical utility in genetic medicine is rarely uniformly defined nor necessarily directly tied to a specific health outcome. As such, generating and evaluating evidence of clinical utility is complex. The challenge in defining clinical utility today is compounded by the extraordinary heterogeneity of rare diseases, as well as the polygenic nature of more common conditions for which WGS is expected to be relevant. In this paper, we aim to extend earlier conceptualizations of clinical utility as applied to the diagnostic use of WGS and suggest that this framework not only be used as a tool for evidence review
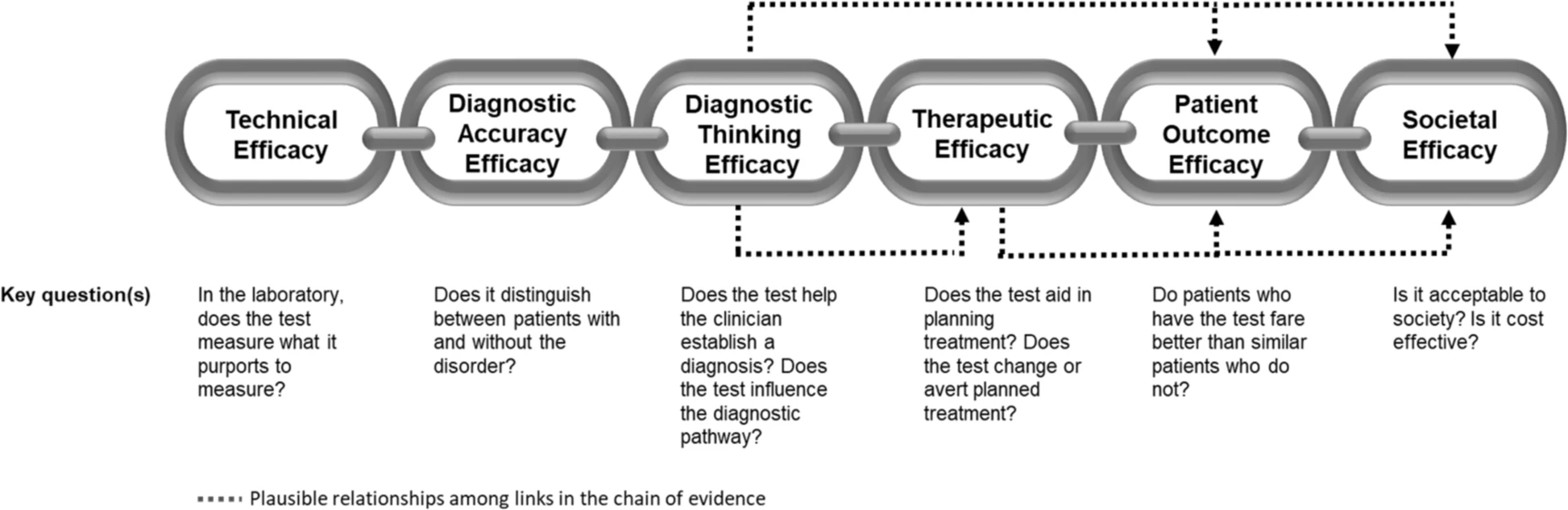
The application of this model to WGS includes six levels of efficacy: technical efficacy, diagnostic accuracy efficacy, diagnostic thinking efficacy, therapeutic efficacy, patient outcome efficacy, and societal efficacy (Table 1, Fig. 1). The model is hierarchical; achieving a given level of efficacy is often but not always contingent upon a demonstration of efficacy at the preceding level. As described in Fig. 1, levels 1–3 are necessarily contingent but beyond level 3, a genetic test can achieve therapeutic, patient outcome, and/or societal impact in ways that are contingent upon one another or independent of one another. We retain the levels of technical and diagnostic accuracy efficacy (i.e., levels 1 and 2) as essential starting points in our guiding framework as they are fundamental precursors to achieving clinical utility. However, since these laboratory-based components of efficacy are well-debated and described in the WGS literature and in recent guidelines published by members of our group27, we focus here on four levels of the efficacy model (i.e., levels 3–6) that align most directly with a broad definition of clinical utility and extend beyond laboratory-based components of efficacy. In emphasizing these four levels of efficacy as components of clinical utility, our intent is to encourage the use of a broad set of health and non-health-related indicators of value to bolster the state of evidence in this area, rather than to convey that all aspects of clinical utility need to be achieved for WGS adoption and reimbursement.
18 de gener 2021
CRISPR 2020, a breakthrough year
2020 Was the Turning Point for CRISPR
At an Oregon hospital in March, a patient with a type of inherited blindness became the first to receive a gene-editing injection directly into their eye. It was the first time CRISPR was used in an attempt to edit a gene inside someone’s body. A second person this year also received the experimental treatment, which is designed to snip out a genetic mutation responsible for their severe visual impairment.
Despite its versatility, CRISPR is still error-prone. For the past few years, scientists have been working on more precise versions of CRISPR that are potentially safer than the original. This year, they made notable progress in advancing these new versions to human patients.
17 de gener 2021
16 de gener 2021
15 de gener 2021
Precision medicine
Precision Medicine for Investigators, Practitioners and Providers
Many topics under the same umbrella:
Table of Contents
Introduction
2. Role of genomics in precision medicine
3. High throughput omics in the precision medicine ecosystem
4. Infant gut microbiome
5. Paraprebiotics
6. Fecal transplantation in autoimmune disease
7. Drug pharmacomicrobiomics
8. CRISPR technology for genome editing
9. Engineering microbial living therapeutics
10. Organ on a chip
11. Multicellular in-vitro organ systems
12. The role of biobanks in biomarker development
13. Translational interest of immune profiling
14. Organoid pharmacotyping
15. Large datasets for genomic investigation
16. Modern applications of neurogenetics
17. Genomic profiling in cancer
18. Genomics in pediatrics
19. Genomics of gastric cancer
20. Genomics of prostate cancer
21. MicroRNAs and inflammation markers in obesity
22. MiRNA sequencing for myocardial infarction screening
23. Cell free DNA in hepatocellular carcinoma
24. Non coding RNA in cancer
25. Germline variants and childhood cancer
26. Pharmacogenomics in cancer
27. Proteomic biomarkers in vireoretinal disease
28. Proteomics in respiratory diseases
29. Cardiovascular proteomics
30. Host genetics, microbiome, and inflammatory bowel disease
31. Sampling, Analyzing, and Integrating Microbiome ‘omics Data in a Translational Clinical Setting
32. Omics and microbiome in sepsis
33. Molecular and omics methods for invasive candidiasis
34. Lipid metabolism in colorectal cancer
35. Salivary volatolome in breast cancer
36. immunodiagnosis in leprosy
37. decision support systems in breast cancer
38. Electronic medical records and diabetes phenotyping
39. Clinical signature of suicide risk
40. Machine learning and cluster analysis in critical care
41. Artificial intelligence in gastroenterology
42. Algorithms for epileptic seizure prediction
43. Precision medicine in ophthalmology
44. Phenotyping COPD
45. Lifestyle medicine
46. Precision medicine for a healthier world
47. Aging and clustering of functional brain networks
48. Nutrigenetics
49. Genome editing in reproductive medicine
50. MRI guided prostate biopsy
51. Precision Nutrition
52. Theranostics in precision oncology
53. Precision medicine in daily practice
54. Imaging in precision medicine
55. Organoid for drug screening
56. Printing of personalized medication using binder jetting 3D printer
57. 3 D printing in orthopedic trauma
58. Consumer genetic testing tools in depression
59. The future of wearables
60. Tumor heterogeneity and drug development
61. Smartphone based clinical diagnosis
62. Smartphone biosensing for point of care use
63. Data security and patient protection
64. Blockchain solutions for healthcare
65. Ethical questions in gene therapy
66. Pitfalls of organ on a chip technologies
67. Regulatory issues of artificial intelligence in radiology
68. Academic industrial alliance
69. The future of precision medicine
70. Precision Medicine Glossary
71. Useful internet sites
14 de gener 2021
13 de gener 2021
12 de gener 2021
11 de gener 2021
10 de gener 2021
09 de gener 2021
Business rules
The Six New Rules of Business. Creating Real Value in a Changing World
1. Intangibles drive value, not the balance sheet: Reputation, trust, and other intangibles—not profit—drive business value and cannot be measured in traditional ways
2. Purpose over profits: Businesses serve many objectives beyond shareholder value. Purpose is more than a slogan; it is revealed through how the company operates and the decisions it makes. Taken seriously, the Purpose signals long term goals and helps the company prevail over short-term pressures.
3. Corporate Responsibility is more than your carbon footprint: Corporate responsibility is defined far outside the business gates—extending through the supply chain and ecosystem to an engaged public.
4. Employees are more than 'stakeholders' -- they are the business: Employees are corporate allies that hold businesses accountable, connect social and environmental issues to business priorities and give voice to risk and competitive advantage in a culture of growing inequality and social unrest.
5. Culture is King: Competition for talent and a focus on innovation and the human element take precedence over capital markets.
6. Co-create, don't compete: When the system is at risk, enlist business partners along the supply chain to raise the bar for the industry as a whole, and win.