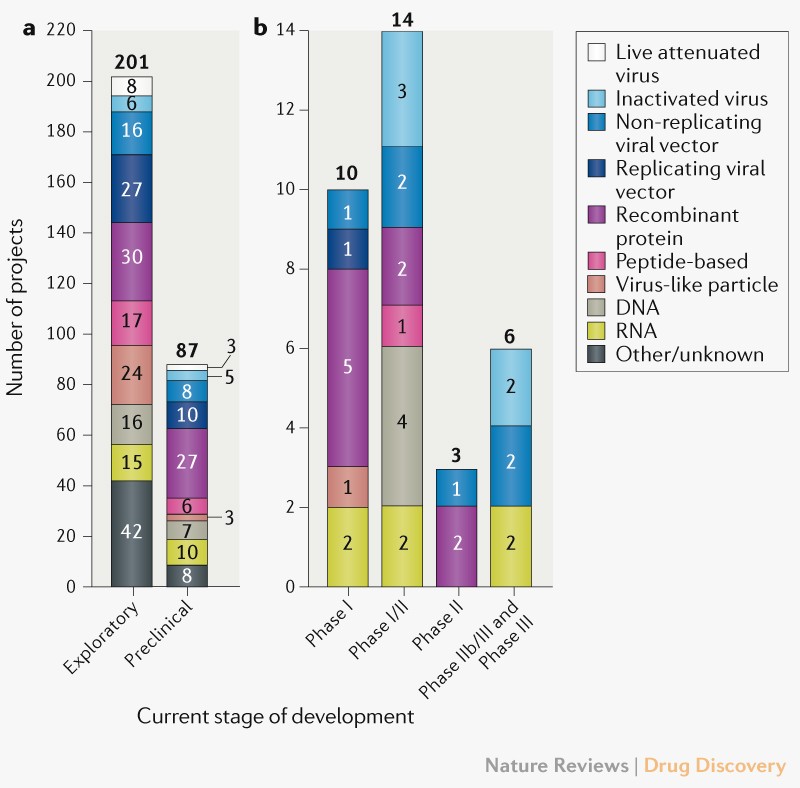
I agree absolutely with Alex Tabarrock and his post in Marginal revolution. He says:
Patents are not the problem. All of the vaccine manufacturers are trying to increase supply as quickly as possible. Billions of doses are being produced–more than ever before in the history of the world. Licenses are widely available. AstraZeneca have licensed their vaccine for production with manufactures around the world, including in India, Brazil, Mexico, Argentina, China and South Africa. J&J’s vaccine has been licensed for production by multiple firms in the United States as well as with firms in Spain, South Africa and France. Sputnik has been licensed for production by firms in India, China, South Korea, Brazil and pending EMA approval with firms in Germany and France. Sinopharm has been licensed in the UAE, Egypt and Bangladesh. Novavax has licensed its vaccine for production in South Korea, India, and Japan and it is desperate to find other licensees but technology transfer isn’t easy and there are limited supplies of raw materials:
Virtually overnight, [Novavax] set up a network of outside manufacturers more ambitious than one outside executive said he’s ever seen, but they struggled at times to transfer their technology there amid pandemic travel restrictions. They were kicked out of one factory by the same government that’s bankrolled their effort. Competing with larger competitors, they’ve found themselves short on raw materials as diverse as Chilean tree bark and bioreactor bags. They signed a deal with India’s Serum Institute to produce many of their COVAX doses but now face the realistic chance that even when Serum gets to full capacity — and they are behind — India’s government, dealing with the world’s worst active outbreak, won’t let the shots leave the country.
Plastic bags are a bigger bottleneck than patents. The US embargo on vaccine supplies to India was precisely that the Biden administration used the DPA to prioritize things like bioreactor bags and filters to US suppliers and that meant that India’s Serum Institute was having trouble getting its production lines ready for Novavax. CureVac, another potential mRNA vaccine, is also finding it difficult to find supplies due to US restrictions (which means supplies are short everywhere). As Derek Lowe said:
Abolishing patents will not provide more shaker bags or more Chilean tree bark, nor provide more of the key filtration materials needed for production. These processes have a lot of potential choke points and rate-limiting steps in them, and there is no wand that will wave that complexity away.
Technology transfer has been difficult for AstraZeneca–which is one reason they have had production difficulties–and their vaccine uses relatively well understood technology. The mRNA technology is new and has never before been used to produce at scale. Pfizer and Moderna had to build factories and distribution systems from scratch. There are no mRNA factories idling on the sidelines. If there were, Moderna or Pfizer would be happy to license since they are producing in their own factories 24 hours a day, seven days a week (monopolies restrict supply, remember?). Why do you think China hasn’t yet produced an mRNA vaccine? Hint: it isn’t fear about violating IP. Moreover, even Moderna and Pfizer don’t yet fully understand their production technology, they are learning by doing every single day. Moderna has said that they won’t enforce their patents during the pandemic but no one has stepped up to produce because no one else can.
More information in his post.
Some weeks ago a journalist asked to me the same question, and I said more or less, the same!. There is no need to start discussions about patents in WCO, only the enforcement and implementation of mandatory licenses can be helpful.
¿Qué opina sobre las patentes de las vacunas de la covid-19? ¿Considera que, en este caso, deberían contemplarse excepciones al derecho de explotación exclusiva?
Antes de hablar de patentes, conviene considerar la inversión pública en investigación. Por ejemplo, en la medida que hay una vacuna cuyo coste de investigación ha sido sufragado en un 97% por el sector público, resulta lógico que se compre a un precio equivalente al coste de fabricación, tal como sucede. Ahora bien, también sería deseable que se obligara a licenciar el proceso a otros fabricantes. En el caso de vacunas de RNA mensajero, el nivel de inversión pública en Estados Unidos es notable y sin embargo no ha sucedido lo mismo. Por consiguiente, los gobiernos deben gestionar las contrapartidas de la inversión pública en investigación.
• ¿Considera que sería positiva una liberación de las patentes de las vacunas contra la covid? ¿Por qué?
En mi opinión ya existen mecanismos que permiten conseguir que las vacunas sean asequibles y son las licencias obligatorias. Tal regulación que se configuró en la reunión de la OMC en Doha en el año 2003. Desafortunadamente no se ha desarrollado suficientemente por los países. Las condiciones por las que se deberían aplicar tales licencias quedan explícitas en la Declaración. Tales condiciones hacen referencia a la definición de emergencia y crisis de salud pública. En esta pandemia se daban las condiciones para su aplicación. Visto así, el debate necesita centrarse entre patentes y licencias obligatorias atendiendo a condiciones concretas.
• ¿Existen mecanismos ya reglados para que, en situaciones como ésta, más allá de la patente, se garantice la llegada de las vacunas a todos los países (incluyendo los de nivel económico más bajo)?
En realidad la Alianza Mundial para vacunas e inmunización (GAVI) nació para ello. En el caso de la COVID, la OMS a través de GAVI y otras instituciones ha impulsado la iniciativa COVAX que pretende ofrecer vacunas a países en desarrollo. Aún así sabemos que el esfuerzo es insuficiente a la vista de los resultados, el 87% de las vacunas ha ido a países ricos, y en los menos desarrollados tan solo ha llegado el 0,2%.