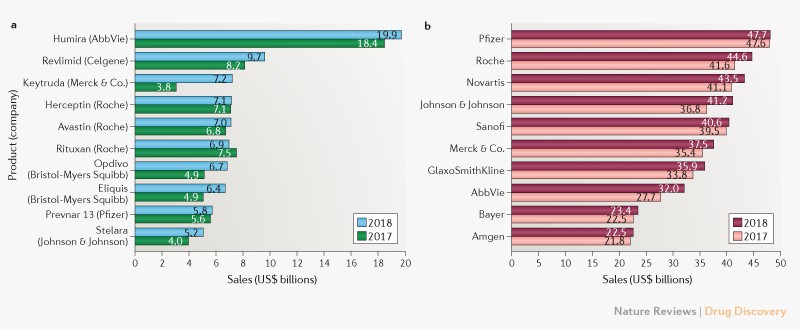
Is there any measure for unfair pricing in drugs?. According to Ezequiel Emanuel prices should not
"exceed 11 percent of the average American’s disposable income. This suggests that current prices for many drugs are excessive and unjust."Why?.
Currently, average lifetime costs for health care are estimated at 31 percent of disposable income. Drugs account for 17 percent of health care expenses. A threshold for medical care as a share of disposable income that is set 10 percentage points higher than the current average amount spent on medical care (at 41 percent, or $261,907) is generous, as is a threshold for drug costs as a share of medical costs set 10 percentage points higher (at 27 percent, or $70,715) than the current share. Using these standards, the costs for all of the drugs a person takes in a lifetime should not consume more than 27 percent of medical costs, or $70,715. This constitutes 11 percent of lifetime disposable income.He achieves this conclusion after applying these principles:
1. Complete life. The unit of analysis should not be a year or other limited time frame, but rather the impact over a whole lifetimeThis article represents a deep change of perspective on drug pricing. Cost-effectiveness of individual drugs are not enough, a lifetime and societal perspective is necessary. I agree in this part, however methodological implications are huge and uncertain.
2. Limited resources. The just price of a drug should reserve enough resources for people to pursue valuable life activities
3. Value. There should exist a close relationship between the actual benefits of an intervention and its price
4. Comprehensiveness. Life activities other than health matter; in considering the benefits of a treatment, we should also consider how it affects education, employment, and other valuable life activities
Bonnard at Tate modern right now