Precision Medicine for Investigators, Practitioners and Providers
Many topics under the same umbrella:
Table of Contents
Introduction
2. Role of genomics in precision medicine
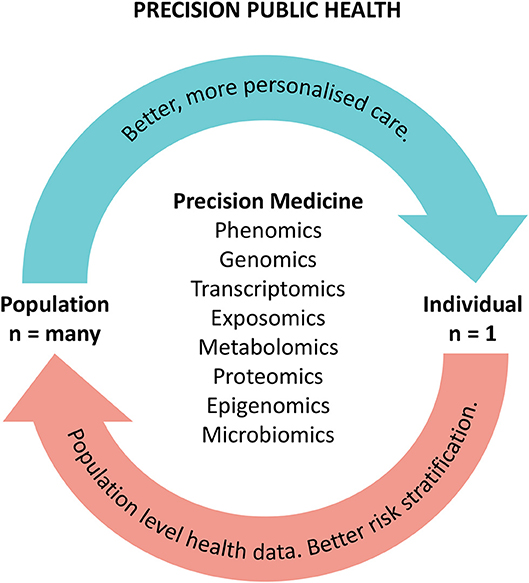
3. High throughput omics in the precision medicine ecosystem
4. Infant gut microbiome
5. Paraprebiotics
6. Fecal transplantation in autoimmune disease
7. Drug pharmacomicrobiomics
8. CRISPR technology for genome editing
9. Engineering microbial living therapeutics
10. Organ on a chip
11. Multicellular in-vitro organ systems
12. The role of biobanks in biomarker development
13. Translational interest of immune profiling
14. Organoid pharmacotyping
15. Large datasets for genomic investigation
16. Modern applications of neurogenetics
17. Genomic profiling in cancer
18. Genomics in pediatrics
19. Genomics of gastric cancer
20. Genomics of prostate cancer
21. MicroRNAs and inflammation markers in obesity
22. MiRNA sequencing for myocardial infarction screening
23. Cell free DNA in hepatocellular carcinoma
24. Non coding RNA in cancer
25. Germline variants and childhood cancer
26. Pharmacogenomics in cancer
27. Proteomic biomarkers in vireoretinal disease
28. Proteomics in respiratory diseases
29. Cardiovascular proteomics
30. Host genetics, microbiome, and inflammatory bowel disease
31. Sampling, Analyzing, and Integrating Microbiome ‘omics Data in a Translational Clinical Setting
32. Omics and microbiome in sepsis
33. Molecular and omics methods for invasive candidiasis
34. Lipid metabolism in colorectal cancer
35. Salivary volatolome in breast cancer
36. immunodiagnosis in leprosy
37. decision support systems in breast cancer
38. Electronic medical records and diabetes phenotyping
39. Clinical signature of suicide risk
40. Machine learning and cluster analysis in critical care
41. Artificial intelligence in gastroenterology
42. Algorithms for epileptic seizure prediction
43. Precision medicine in ophthalmology
44. Phenotyping COPD
45. Lifestyle medicine
46. Precision medicine for a healthier world
47. Aging and clustering of functional brain networks
48. Nutrigenetics
49. Genome editing in reproductive medicine
50. MRI guided prostate biopsy
51. Precision Nutrition
52. Theranostics in precision oncology
53. Precision medicine in daily practice
54. Imaging in precision medicine
55. Organoid for drug screening
56. Printing of personalized medication using binder jetting 3D printer
57. 3 D printing in orthopedic trauma
58. Consumer genetic testing tools in depression
59. The future of wearables
60. Tumor heterogeneity and drug development
61. Smartphone based clinical diagnosis
62. Smartphone biosensing for point of care use
63. Data security and patient protection
64. Blockchain solutions for healthcare
65. Ethical questions in gene therapy
66. Pitfalls of organ on a chip technologies
67. Regulatory issues of artificial intelligence in radiology
68. Academic industrial alliance
69. The future of precision medicine
70. Precision Medicine Glossary
71. Useful internet sites