Li pregunto a Chat GPT sobre l'assaig clínic més gran en nombre de participants i em diu que és ASPREE, sobre l'aspirina i prevenció cardiovascular. I veig que Galleri, l'assaig clínic de GRAIL sobre cribratge de càncer al NHS hi ha 140.000 participants. I jo penso, això és molta gent i el Chat GPT4 no ho ha copsat.
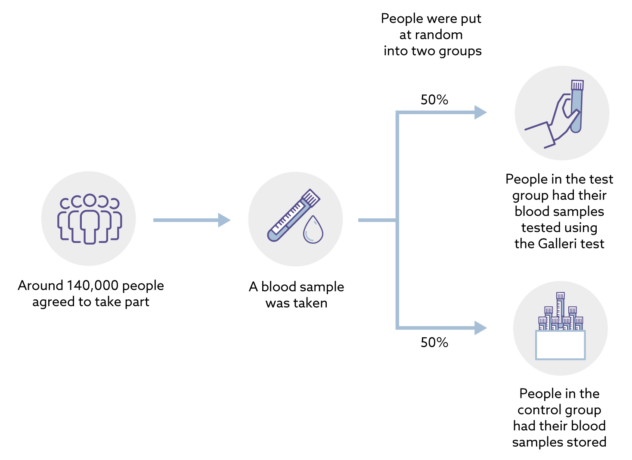
This is a trial in a large population in England to assess the performance and clinical utility of GRAIL’s multi-cancer early detection test when added to standard of care. Participants are randomized to either a test or control arm. The study teams remain blinded throughout the study. Participants who test positive will be referred for standard of care investigations and treatment in the National Health Service (NHS).
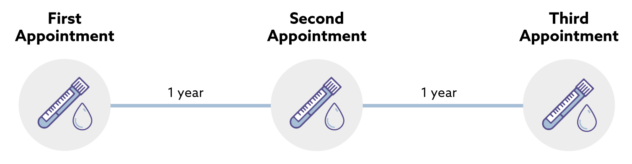
The study has enrolled approximately 140,000 people aged 50 to 77. Unless diagnosed with cancer, participants in both arms are asked to return for annual visits at approximately 12 and 24 months. All participants whether test positive, test negative or not tested will be followed for cancer and associated outcomes via linkage to NHS routine datasets.
Els detalls de l'assaig els trobareu aquí. I si voleu veure la validació, aquí. I mentrestant el regulador segueix pensant si autoritza la prova, resulta que ja es troba al mercat.
Health information, such as whether someone developed cancer and how it was treated, is collected from centrally held NHS records for up to 10 years after people’s first appointment. This allows the researchers to easily track people for whether they get cancer, even for people who may have moved home.People who are diagnosed with cancer while they are taking part in the trial may not need to attend further trial appointments to give blood samples.
A quina data s'acaba l'assaig clínic no se sap del cert. Després de llegir tot això espero que se sàpiga quina és la sensibilitat i especificitat de la prova segons càncer detectat. Dic això perquè no he sabut veure com s'avaluen els falsos negatius, ni els falsos positius. I en un moment concret diuen que haurem d'esperar 10 anys. No ho sé, potser m'ha passat per alt.
Més detalls, aquí.
Mentrestant la Unió Europea ha prohibit la fusió Illumina-Grail, i ja veurem com acaba. Per ara els mesos passen i res de res...
Galleri, el test detecta (diuen) 50 tipus de càncer amb una gota de sang que cerca ADN circulant per 1.000€ (diuen, diuen).
Més d'un i més de dos volen saber si estem davant d'un nou cas Theranos, (jo vull pensar que no) i per això han començat aquest assaig clínic. A FT del dijous hi ha més detalls. I ho fan a UK perquè a Europa aquesta empresa hauria d'estar prohibida fins que no compleixi les resolucions de les autoritats.