07 de novembre 2014
Fasten seat belts (4)
Any physician asking for hepatitis C drugs will have to explain the compliance with the criteria and ask for approval.
I said some weeks ago that a new paradigm in drug pricing was starting, right now I have to say that drug prescription priorisation by rules is the new trending topic, at least in our neighbourghood. Wether this prioritisation is based by cost-effectiveness criteria remains to be seen.
16 d’abril 2025
Pooling plasma, quin desastre! (2)
El llibre "Blood on Their Hands: How Greedy Companies, Inept Bureaucracy, and Bad Science Killed Thousands of Hemophiliacs" d'Eric Weinberg i Donna Shaw, publicat el 2017, documenta la tragèdia de com milers de persones amb hemofília van resultar infectades amb el VIH a causa de productes sanguinis contaminats, assenyalant com a principals responsables a empreses avaricioses, una burocràcia incompetent i una ciència deficient.
El llibre es basa en la investigació d'Eric Weinberg, un advocat que va representar a moltes de les víctimes i les seves famílies. La prefaci comença amb el funeral de Joe Salgado, un amic de Weinberg que va morir a causa de la infecció per VIH adquirida per tractaments per a l'hemofília, establint la connexió personal de l'autor amb la comunitat afectada.
La taula de continguts revela l'estructura narrativa del llibre, que abasta des dels orígens de la producció de productes sanguinis fins a les conseqüències legals i personals de l'epidèmia.
- La secció "Beginnings" (Capítol 2) probablement explora la història primerenca del tractament de l'hemofília i el desenvolupament dels concentrats de factor.
- "How Could It Happen and Nobody Did Anything Wrong?" (Capítol 3) planteja la qüestió central de la responsabilitat.
- "A History Ignored" (Capítol 4) examina el context històric, possiblement detallant advertències i riscos que es van ignorar. Es menciona que es va identificar un sistema de donació de sang totalment voluntari com una de les millors maneres de reduir el risc, però va trobar resistència per part de gairebé tothom. També es discuteix com la comunitat d'hemofílics va començar a veure les implicacions de donar la iniciativa a la indústria en lloc dels bancs de sang i hospitals. Robert Massie, autor d'un llibre sobre la criança d'un fill amb hemofília, inicialment tenia grans esperances que la Creu Roja Americana produís concentrats de Factor VIII, però això no va passar.
- "Digging In" (Capítol 5) descriu la investigació de Weinberg, que va incloure la descoberta de documents importants, com ara un informe de 1976 titulat "Unsolved Therapeutic Problems in Hemophilia" que va organitzar el Dr. Aronson, considerat crític per al cas. Aquest informe abordava un virus desconegut anomenat hepatitis no-A, no-B, que es transmetia als pacients amb hemofília. Weinberg també va decidir anar a Alemanya per obtenir més informació sobre Behringwerke.
- "Reaching Out" (Capítol 6) narra els esforços de Weinberg per connectar amb altres advocats i possibles aliats en la lluita legal, com David Shrager.
- "Help Wanted" (Capítol 7) tracta sobre la cerca d'ajuda i recursos per al cas, incloses les interaccions amb l'Associació d'Hemofília de Nova Jersey (HANJ) i la seva directora executiva, Elena Bostick. HANJ va acceptar una oferta de patrocini d'un seminari amb la condició que s'hi convidés a tots els advocats involucrats en litigis d'hemofília a Nova Jersey, que en aquell moment era només Weinberg.
- "All for Business" (Capítol 8) explora com els interessos comercials van influir en les decisions relacionades amb la seguretat dels productes sanguinis. Weinberg va contactar amb el Dr. Shanbrom, que tenia patents relacionades amb l'hemofília, per investigar si hi havia tecnologia disponible per netejar els productes de factor abans que el VIH contaminés el subministrament de sang.
- "Somewhere Here, I Have the Documents" (Capítol 9) se centra en la dificultat d'obtenir proves i documents clau.
- "More Lawyers, More Experts" (Capítol 10) detalla la construcció de l'equip legal i la cerca de testimonis experts. Weinberg es va reunir amb el Dr. Dubin per revisar informació sobre els seus clients. També va haver de buscar en la literatura mèdica referències a la necessitat de netejar els fàrmacs de coagulació. Va trobar un article clau al New England Journal of Medicine de 1978 que alertava sobre malalties hepàtiques en hemofílics i instava la indústria a netejar els concentrats. El Dr. Van Thiel, un dels autors, va acceptar ser un testimoni expert.
- "A Meeting with Roger" (Capítol 11) descriu trobades i estratègies legals.
- "An Act of Man" (Capítol 12) probablement examina les accions i omissions individuals que van contribuir a la tragèdia.
- "The Trouble with Torts" (Capítol 13) aborda les complexitats del dret de danys en aquest tipus de litigis.
- "I Murdered My Child, But Not Alone" (Capítol 14) ofereix una perspectiva personal i devastadora de les conseqüències de la contaminació.
- "Of Sheep and Men" (Capítol 15) i "A Failure of Leadership" (Capítol 16) analitzen la responsabilitat de la indústria i les autoritats reguladores. Es menciona que Baxter utilitzava plasma recollit de presons, incloent-hi la Penitenciaria Estatal de Louisiana a Angola. També es detalla com els executius d'Armour van decidir no notificar a la FDA sobre les dades del Dr. Prince i van continuar venent Factorate. El govern federal estava preocupat pel risc d'hepatitis d'un producte de coagulació sanguínia de Hyland ja el 1968, però la companyia no entenia la preocupació. Behringwerke AG a Alemanya estava provant un producte de coagulació tractat amb calor el 1979, cosa que va fer que Baxter tornés a posar la investigació sobre l'hepatitis a la seva llista de prioritats "A".
- "From Prime Chuck to Dogeza" (Capítol 17) i "Endings" (Capítol 18) narren les conclusions dels processos judicials i les seves resolucions. Es menciona l'aprovació de la llei Ricky Ray Hemophilia Relief Fund Act per la Cambra de Representants dels EUA.
- L'epíleg ofereix reflexions finals sobre la tragèdia i les seves implicacions per a la seguretat del subministrament de sang en l'actualitat.
En resum, "Blood on Their Hands" és un relat detallat de la crisi de la contaminació per VIH dels productes de factor VIII utilitzats per tractar l'hemofília als Estats Units. El llibre exposa com els guanys empresarials van prevaldre sobre la seguretat del pacient, com la ineptitud burocràtica no va poder prevenir la propagació de la infecció i com la mala ciència i la manca de diligència van contribuir a la mort de milers de persones. A través de la investigació de Weinberg i les històries personals de les víctimes, el llibre ofereix una acusació contundent de les falles sistèmiques que van conduir a aquesta tragèdia.
24 d’abril 2015
A successful implementation of a bad idea
This is really a bad idea that is already being implemented. As you know sometimes there are good ideas badly implemented, and therefore criticized. But in this case, it is a bad idea with a scrupulous implementation. Some officials consider that if they set a budget ceiling, all decisions will be taken to fit in with it. Clinical decisions follow a different path, not the mechanical and administrative way officials are used to.
The measure represents a tough hit to economic evaluation, because in the next future the government will not be any longer interested in it. Why? Their only concern is about the budget ceiling, the value doesn't matter. A missed opportunity for the development of priority setting under a rational scheme. Health economists should react to such a big mistake.
The saddest issue is that nobody knows what will happen when the budget ceiling is surpassed. This will be the job for the next government, nobody cares about it right now. Democracy and rule of law are only words subject to interpretation.
PS. All the details about hepatitis C controversy at Boletín AES.
PS. Understanding the foundations of confidential drug pricing, in Forbes.
PS. Explained at Health Affairs:
31 de març 2025
Pooling plasma, quin desastre!
Blood Farm. The Explosive Big Pharma Scandal that Altered the AIDS Crisis
Resumit amb IA
El llibre "Blood Farm " detalla la tragèdia i l'escàndol de la sang contaminada, principalment relacionada amb l'ús del Factor VIII per al tractament de l'hemofília, i la seva contaminació amb virus com el VIH (causant de la SIDA) i l'hepatitis C. El llibre explora com milers de persones amb hemofília al Regne Unit i als Estats Units van resultar infectades per aquests virus a causa de productes sanguinis contaminats, i com les empreses farmacèutiques, els metges i els governs van gestionar la crisi, sovint amb negligència i encobriment.
El llibre presenta diverses perspectives i línies argumentals:
- Els orígens i la naturalesa del problema: El Factor VIII, derivat de plasma sanguini de múltiples donants, es va convertir en un tractament revolucionari per a l'hemofília. No obstant això, el procés de "pooling" de plasma de milers de donants va fer que una sola donació infectada pogués contaminar lots sencers de producte. La falta de proves de detecció viral adequades i la lentitud en l'adopció de processos d'inactivació viral, com el tractament tèrmic, van agreujar el problema.
- La propagació de la informació i la reacció inicial: A principis dels anys 80, van començar a sorgir informes sobre una possible "malaltia misteriosa" (SIDA) que afectava persones amb hemofília que havien rebut productes sanguinis nord-americans. Tot i les advertències i les evidències creixents sobre el risc de transmissió del VIH i l'hepatitis a través del Factor VIII importat dels EUA, les autoritats sanitàries del Regne Unit van ser lentes a reaccionar i a advertir adequadament els pacients.
- El paper de les institucions i els individus clau:
- Treloar's School: El llibre explora el cas de Treloar's, una escola per a nens amb discapacitats físiques on molts estudiants amb hemofília van ser tractats amb Factor VIII contaminat i potencialment utilitzats en assaigs clínics sense consentiment informat. El doctor Aronstam, responsable del centre d'hemofília de l'escola, va tenir un paper central en el tractament dels nens i en la recopilació de dades.
- Doctors i hematòlegs: Figures com el Professor Bloom a Cardiff van començar a reconèixer el vincle entre el Factor VIII i la SIDA. Tot i algunes veus que alertaven sobre els riscos, hi va haver una reticència inicial per part de la comunitat mèdica i les autoritats a acceptar i comunicar la magnitud del problema.
- Pacients i famílies: El llibre narra les històries personals de pacients com Ade i Bryan, que van resultar infectats amb VIH a través del tractament amb Factor VIII. També es destaca la lluita de famílies com els Cross per buscar justícia per la infecció del seu fill Brad.
- Advocats i activistes: Tom Mull, un advocat, va tenir un paper crucial en la investigació i la presentació de demandes contra les companyies farmacèutiques als EUA. Activistes com Dana Kuhn van treballar per exposar la veritat i buscar responsabilitats.
- Periodistes: Sue Douglas del Mail on Sunday va ser una de les primeres a portar la història de la "sang assassina" a l'atenció pública al Regne Unit.
- La resposta de les companyies farmacèutiques: El llibre exposa com companyies com Alpha, Armour, Baxter i Bayer van continuar venent Factor VIII potencialment contaminat fins i tot quan van sorgir proves dels riscos. Es detallen pràctiques com la recollida de plasma en presons (incloent Angola a Louisiana) i d'altres fonts d'alt risc, i la continuació de la venda de productes no tractats tèrmicament quan ja hi havia alternatives més segures disponibles. També es revela com algunes companyies van prioritzar els beneficis econòmics per sobre de la seguretat dels pacients, fins i tot intentant encobrir els riscos.
- Les batalles legals i la recerca de justícia: El llibre segueix les complexes batalles legals als Estats Units, com el cas de la família Cross contra Cutter, on les companyies van intentar negar la seva responsabilitat. També es menciona la situació a França i al Canadà, on es van prendre mesures diferents.
- La investigació i les revelacions posteriors: El llibre fa referència a la Infected Blood Inquiry al Regne Unit, una investigació pública exhaustiva que va començar molts anys després per examinar a fons l'escàndol i les responsabilitats. Es destaca la importància de documents interns de les companyies i del govern que van ser descoberts i que revelaven el coneixement que tenien dels riscos i la seva resposta.
- L'impacte a llarg termini i la recerca de tancament: El llibre explora les conseqüències devastadores per a les persones infectades i les seves famílies, incloent la malaltia, l'estigma i la mort. També es reflexiona sobre la dificultat de trobar un tancament per a aquesta tragèdia i la importància que es faci justícia i s'aprenguin les lliçons per al futur.
En resum, "Blood Farm" és una investigació profunda i emotiva sobre un dels pitjors desastres sanitaris de la història, que revela la negligència, l'avarícia i l'encobriment que van portar a la infecció de milers de persones amb hemofília, i la llarga i difícil lluita per la veritat i la justícia per part de les víctimes i les seves famílies.
PS. Avui, Gutierrez-Rubí a LV, encertat
02 d’octubre 2014
Fasten seat belts
In my opinion, such a situation allows to understand better that the pharmaceutical market for innovative drugs is mostly a monopsony (one buyer) in a monopoly (one seller), it is not a competitive market - and this is what I have always considered. Therefore, resource allocation is the result of a bargaining between both parties, and the unit price is irrelevant. The buyer wants to maximize health, the seller is maximizing income, this is exactly the struggle.
The key question is: How much is NHS willing to pay for better health?. As far as the budget is limited, the number of treatments times the price is not the right way to proceed to maximize health under constrained resources.
Any government has to set priorities for expenditure according to expected health value created. This information should be public. In any case, when a new drug is available the government should clearly define which benefits are cancelled and which are acceptable. A responsible minister can't agree new expenditures without any budget.
Therefore, innovative pharmaceutical market is not really a market -right now is clear- and governments should set priorities according to resources available -right now is also clear that they haven't done it-.
Fasten seat belts, we are entering into trying times without any political compass-gps. Citizens are expecting something different. I still remember when Victor Fuchs told long time ago: usually health economists discuss incremental cost-effectiveness in limited marginal terms, the real issue appears when such an amount is enormous. The case of hepatitis C is the example of such a situation, and only health policy and deliberative democracy are the tools to confront it. Unfortunately, this was not the strategy applied nearby.
PS. Catalonia in contention, at Harvard Political Review. Must read, if you are interested on what's going on. Otherwise, try Bloomberg op-ed or LAtimes.
PS. Reading Francesc-Marc Alvaro op-ed I always learn something.
PS. Rating catalans' well-being by OECD.
08 de juny 2025
El mercat farmacèutic tensionat
Economic Markets and Pharmaceutical Innovation
Aquí teniu un resum detallat de l'article "Economic Markets and Pharmaceutical Innovation" de Craig Garthwaite, basant-me en la informació proporcionada a les fonts:
L'article, publicat al Journal of Economic Perspectives, ofereix un resum ampli del coneixement existent sobre diversos aspectes econòmics importants del mercat farmacèutic relacionats amb el procés innovador. L'autor és Craig Garthwaite, Professor d'Estratègia a la Kellogg School of Management de la Northwestern University. L'objectiu és ajudar aquells interessats o involucrats en debats sobre la indústria farmacèutica a entendre els fets i patrons econòmics fonamentals al voltant dels incentius per al desenvolupament de medicaments.
L'article comença assenyalant una tensió central en els mercats farmacèutics: tot i que els beneficis dels fàrmacs innovadors són poc discutits, molts pregunten raonablement "A quin preu?". Les enquestes d'opinió pública mostren una favorabilitat neta negativa per a la indústria farmacèutica, tot i que els fàrmacs innovadors han tingut un èxit enorme en el tractament i la curació de diverses condicions. Per exemple, les innovacions farmacèutiques són responsables del 35% de la notable disminució de la mortalitat cardiovascular del 1990 al 2015. Condicions prèviament mortals com el VIH/SIDA s'han transformat en malalties cròniques manejables, i l'hepatitis C s'ha curat. Teràpies gèniques i avanços en immuno-oncologia estan proporcionant millores significatives. Recentment, han aparegut tractaments efectius per a l'obesitat (agonistes GLP-1) amb millores en resultats cardiometabòlics.
Els preus elevats dels medicaments de marca innovadors no són accidentals, sinó el resultat de polítiques destinades a proporcionar incentius financers per al desenvolupament continu de nous productes. Aquestes polítiques són necessàries a causa d'una fallada de mercat intrínseca a la innovació farmacèutica. Les empreses que desenvolupen productes nous creen béns públics en forma de coneixement científic. Un cop desenvolupat aquest coneixement, és relativament fàcil per a una altra empresa replicar el producte a un cost fix molt menor i reduir el rendiment financer per a l'innovador original. Per tant, sense protecció de la propietat intel·lectual, poques empreses racionals invertirien els grans costos i riscos necessaris per a la innovació, especialment amb llargs períodes de retorn i baixos costos d'imitació. El sistema actual implica que els governs proporcionin protecció de la propietat intel·lectual per períodes limitats, permetent a les empreses innovadores vendre els seus productes a un preu més alt sense competència directa.
Aquesta protecció de la propietat intel·lectual crea un equilibri entre accés a curt termini i incentius a llarg termini. Els preus elevats redueixen l'accés a les innovacions existents a curt termini, cosa que pot tenir conseqüències devastadores per als pacients. Tanmateix, aquests preus elevats proporcionen incentius financers per a futures inversions en el desenvolupament de nous productes, que podrien resoldre la manca de tractaments per a condicions que actualment no en tenen.
El desenvolupament de productes farmacèutics innovadors requereix inversions grans, fixes i enfonsades en R&D (recerca i desenvolupament). Aquests fons provenen d'empreses privades, universitats i institucions públiques. Molts productes exitosos comencen en laboratoris acadèmics i petites firmes de biotecnologia. La inversió del sector privat en R&D farmacèutic va ser de 276.000 milions de dòlars a nivell mundial el 2021.
Nombrosos estudis empírics mostren una relació entre la mida esperada del mercat d'un fàrmac i la magnitud de les inversions en R&D. Les firmes responen a la mida econòmica potencial del mercat d'un producte, no només al nombre de pacients potencials. Per exemple, l'expansió de Medicaid als EUA va tenir un augment modest en els ingressos a causa dels preus més baixos que paga Medicaid, i no va resultar en un augment de les inversions en R&D. Les tàctiques de negociació més fortes, com l'exclusió de cobertura, van reduir el retorn financer per pacient potencial i van disminuir les inversions en R&D en àrees amb substituts terapèutics competidors. La diferència de preus entre els EUA i altres mercats desenvolupats suggereix que molts altres mercats són probablement massa petits per ser determinants en les decisions d'inversió en desenvolupament de fàrmacs de les firmes innovadores, cosa que explica per què poden negociar preus molt més baixos sense grans temors de reduir la innovació futura.
El benefici exacte creat per aquestes inversions incrementals en R&D no està clar. La quantitat de despesa en R&D o el nombre de productes nous són mètriques incompletes del benefici social. Els estudis sobre la novetat científica dels fàrmacs marginals donen resultats contradictoris. Alguns estudis suggereixen que els augments de la mida del mercat per polítiques com Medicare Part D van portar a productes que no eren científicament nous en el seu enfocament bàsic, sinó noves combinacions d'enfocaments existents. Altres troben que les firmes amb grans infusions de caixa per polítiques com Medicare Part D van augmentar les inversions en productes més científicament nous, independentment dels retorns potencials, suggerint que les infusions de caixa van reduir les friccions en el mercat financer. Aquests resultats suggereixen que promoure la innovació més científica pot requerir abordar impediments específics com l'aversió al risc o la manca de ciència bàsica. La novetat científica no és suficient per encapsular els efectes del benestar dels nous productes; les noves aplicacions de mecanismes d'acció existents també poden tenir impactes significatius en la salut i el benestar econòmic.
La determinació dels ingressos potencials per a un nou producte farmacèutic depèn de qui és el client, quin és exactament el "preu" i la mida esperada del mercat. Això requereix comprendre el mecanisme d'establiment de preus, els determinants de la demanda i els factors institucionals que els influeixen.
La cadena de valor farmacèutica implica tres tasques perquè una innovació tingui èxit comercial: (1) aprovació per un regulador, (2) acceptació de pagament per un tercer pagador, i (3) adopció per part dels clínics i pacients.
Hi ha dues cadenes de valor principals:
- Fàrmacs al detall ("retail pharmaceuticals"): Prescrits per un metge i comprats a una farmàcia. La relació entre els tercers pagadors (asseguradores), les firmes gestores de beneficis farmacèutics ("pharmacy benefit managers" o PBMs) que contracten, i els fabricants de medicaments és crucial. Els productes entren amb un preu de llista ("list price"). El PBM negocia un descompte amb el fabricant, conegut com a "rebate" (rebaixa), que varia segons el poder de negociació. Els rebaixes es mantenen confidencials per facilitar descomptes més grans. Els PBMs i els fabricants també negocien la gestió de la utilització, on majors descomptes resulten en menys eines com autoritzacions prèvies o teràpia esglaonada. La gestió de la utilització també pot incloure eines financeres com deduccibles, coassegurança i copagaments. Els PBMs obtenen ingressos de quotes per membre per mes, un percentatge de la rebaixa negociada i el diferencial entre el que paguen a la farmàcia i el que el patrocinador del pla els paga ("spread pricing"). La compensació dels PBMs, especialment lligada a les rebaixes, genera controvèrsia.
- Fàrmacs administrats per metges ("physician-administered drugs"): Sovint són productes biològics administrats en un consultori mèdic. Aquests es paguen sota la porció "mèdica" del benefici de l'assegurança i no involucren PBMs. Els proveïdors sanitaris els adquireixen, emmagatzemen i administren. Se'ls sol pagar un marge sobre el preu mitjà de venda del producte. A Medicare, els metges reben el 106% del preu mitjà de venda, mentre que els mercats privats solen tenir marges més grans. Aquesta forma de pagament pot distorsionar l'entrada i la difusió de nous productes, ja que els metges reben més ingressos per prescriure productes de major preu. Això ha estat una preocupació política per als incentius al desenvolupament de biosimilars. Els proveïdors més grans poden negociar descomptes més grans, cosa que pot influir en les decisions de consolidació de les firmes. Aquesta cadena de valor també pot generar distorsions, ja que les decisions de cobertura d'un pagador poden influir en la disponibilitat del fàrmac per a pacients coberts per altres pagadors.
Les negociacions determinen el preu capturat pels fabricants. Les rebaixes han crescut en magnitud i importància estratègica. L'escletxa entre el preu de llista i el preu net per a fàrmacs al detall es va mantenir relativament estable del 2009 al 2013, però després el preu de llista va escalar ràpidament mentre que el preu net va romandre constant. El 2019, la magnitud de les rebaixes es va duplicar i la mitjana era aproximadament del 53%. El preu de llista i la rebaixa depenen de l'entorn competitiu. Teràpies relativament úniques tenen preus nets més alts que s'apropen més als preus de llista. L'entrada de productes competidors, com va passar amb els tractaments per a l'hepatitis C, pot portar a fortes negociacions i disminucions de preus. L'entrada de nous productes pot estendre's més enllà de l'augment de la competència; les segones i terceres generacions de fàrmacs poden proporcionar una eficàcia superior, menys efectes secundaris o ser millors per a grups de pacients específics. En aquests casos, els preus no cauen amb l'entrada, sinó que cada versió del fàrmac tracta una població més petita i específica.
Entendre la disposició a pagar ("willingness-to-pay") per als productes farmacèutics és complex. El guany en salut és un punt de partida lògic, sovint quantificat mitjançant anys de vida ajustats per qualitat (QALYs). Tanmateix, en un mercat amb assegurances, el consum es finança amb recursos de tot el grup de risc, molts dels quals no es beneficien directament de la transacció. Hi ha altres fonts potencials de valor que van més enllà del benefici directe per al pacient, com el valor d'opció per a pacients no afectats, el valor del progrés científic per a tractaments futurs i el valor d'assegurança de la innovació mèdica. Les innovacions mèdiques transformen el risc mèdic en risc financer per als individus. El valor d'assegurança d'una nova innovació pot superar el valor de l'assegurança de salut mateixa, especialment per a malalties greus amb tractaments limitats. Aquest valor d'assegurança podria explicar per què molts tractaments per a malalties rares superen els llindars basats únicament en el valor clínic. El progrés científic és iteratiu, i el valor futur creat per les innovacions futures hauria de ser tingut en compte en els ingressos dels fabricants que fan progrés incremental. Els esforços per vincular els preus dels fàrmacs al valor basat únicament en resultats clínics podrien negligir aquests altres beneficis de la innovació i crear preus artificialment baixos, alterant els incentius per invertir.
Els fabricants intenten augmentar els ingressos potencials mitjançant esforços de comercialització dirigits a pacients i proveïdors. Aquests esforços estan regulats per la FDA. Les empreses només poden publicitar per a indicacions aprovades, tot i que els metges poden utilitzar productes aprovats per a qualsevol indicació. Obtenir l'aprovació per a noves indicacions pot augmentar la informació del mercat i l'ús. Hi ha preocupacions sobre si la despesa en màrqueting és un malbaratament o un complement a la R&D; si augmenta la mida del mercat potencial, pot encoratjar la inversió en desenvolupament de productes.
El màrqueting dirigit directament als consumidors (DTC advertising) només està permès als EUA i Nova Zelanda en mercats de salut. Hi ha debat sobre si és principalment informació o persuasió. Pot informar els pacients sobre possibles tractaments, i estudis troben que pot augmentar l'ús de medicaments genèrics, cosa que seria coherent amb l'augment de visites mèdiques. D'altra banda, en una població assegurada, pacients i proveïdors podrien no ponderar adequadament el valor dels productes si no afronten el cost marginal, cosa que podria portar a l'ús de medicaments poc rendibles. Estudis han trobat que l'ús resultant del màrqueting DTC pot generar guanys per als pacients, com l'augment de l'oferta laboral o una major adherència. L'efecte sobre el benestar depèn dels beneficis clínics del fàrmac anunciat. Alguns estudis suggereixen que les firmes poden ser més propenses a anunciar fàrmacs amb menors beneficis clínics, mentre que altres troben beneficis econòmics significatius per a fàrmacs altament efectius com les estatines, superant la despesa total en publicitat DTC en el mercat.
El màrqueting dirigit als proveïdors ("detailing") implica fabricants dirigint-se als metges. La majoria dels recursos de màrqueting es dirigeixen als metges. La pregunta clau és si aquests esforços augmenten la informació disponible per als metges o només busquen influir en el seu comportament de prescripció mitjançant beneficis tangibles. Estudis suggereixen que els pagaments als metges porten a petits augments en les prescripcions i la despesa dels pacients, generant un retorn econòmicament significatiu per als fabricants. L'impacte en el benestar depèn de per què els pacients no accedien prèviament a medicacions efectives i l'eficàcia de les que acaben prenent. Alguns estudis troben que el màrqueting als metges pot augmentar l'ús tant en pacients d'alt com de baix risc d'esdeveniments adversos, generant escepticisme sobre la precisió de la informació proporcionada. Igual que amb la publicitat DTC, l'anàlisi de benestar per al detailing als metges és complex i depèn de la classe de fàrmacs. Les firmes coordinen els seus esforços de comercialització DTC i dirigits als metges.
Els incentius per a la innovació depenen que les firmes obtinguin marges positius per recuperar les inversions en R&D i regulació. Avaluar els efectes agregats requereix considerar l'impacte de les assegurances. Una assegurança que funcioni bé pot aïllar els pacients del cost directe, reduint les pèrdues de benestar per preus alts. No obstant això, estudis suggereixen que en el mercat nord-americà, altament assegurat, les quantitats de medicaments disminueixen poc després de la pèrdua de patents tot i la caiguda dels preus, indicant un grau limitat en què els preus alts limiten el consum en mercats amb assegurança. Això pot no ser cert en mercats sense assegurança o amb cost-sharing elevat. L'estructura de l'assegurança és important.
L'article descriu tres programes públics d'assegurança de medicaments:
- Medicaid: Cobreix població de baixos ingressos i discapacitada. Utilitza dos mètodes per assegurar els preus més baixos: (1) rebaixes al govern estatal de Medicaid iguals al major del 23,1% del preu de llista o la major rebaixa a qualsevol comprador comercial ("best price"), i (2) rebaixes "inflacionàries" addicionals basades en el creixement del preu de llista des del llançament del producte. Això resulta que Medicaid només paga el 35% del preu mitjà pagat per les asseguradores a Medicare Part D i el mercat comercial. Molts productes amb preus comercials alts es venen a Medicaid pràcticament sense cost. Les firmes reaccionen a aquestes polítiques; la regla del "best price" augmenta els costos per als fabricants de proporcionar descomptes al mercat comercial, actuant com un impost implícit en aquest mercat. Les rebaixes inflacionàries incentiven a limitar el creixement del preu de llista amb el temps.
- El Programa de Preus de Medicaments 340B: Creat el 1992 per subvencionar hospitals que proporcionen atenció no compensada. Permet a certs hospitals comprar productes farmacèutics aproximadament al "best price" de Medicaid. Poden vendre aquests productes a pacients ambulatoris. Quan venen a pacients de Medicaid, han d'acceptar el "best price". Per a pacients comercials o sense assegurança, poden carregar els preus que negociïn, però una gran fracció no trasllada els descomptes a pacients sense assegurança o amb poca assegurança. El programa ha crescut significativament, amb un augment enorme del nombre de farmàcies contractades i del valor dels productes. Té implicacions a nivell de mercat; en reduir els ingressos disponibles per a fàrmacs innovadors, pot disminuir els incentius per a la R&D. Té un efecte similar al de Medicaid en els preus comercials. També pot tenir altres efectes, com reduir l'incentiu a proporcionar rebaixes als plans comercials i, en alguns casos, fer que els patrocinadors dels plans paguin preus de llista més alts. El creixement del 340B s'associa amb augments en les primes d'assegurança de salut comercial. El programa està relativament poc estudiat en comparació amb altres.
- Medicare Part D: Creat el 2006 com un programa d'assegurança de medicaments al detall per a la gent gran. Està molt subvencionat pel govern, però gestionat per firmes privades que negocien preus de manera similar al mercat comercial. Històricament, incloïa un component de reassegurança ("catastrophic region") on el govern assumia la major part de la despesa un cop el pacient assolia un cert límit de despesa de butxaca. L'estructura inicial incentivava tant els plans com els fabricants a augmentar ràpidament els preus de llista, ja que això feia que els pacients de cost elevat entressin més ràpidament a la regió catastròfica coberta principalment pel govern. La Llei de Reducció de la Inflació (IRA) del 2022 va canviar l'estructura de la regió catastròfica, transferint més càrrega als patrocinadors dels plans i eliminant la porció pagada pel pacient. La IRA també va concedir autoritat a CMS per negociar preus més baixos per a fàrmacs seleccionats després de 9 anys (molècules petites) o 13 anys (biològics) de la primera aprovació. Aquest període no s'estén per noves indicacions. Les implicacions de la IRA sobre la innovació encara es debaten i són objecte d'investigació.
El cost compartit estratègic ("strategic cost sharing") en els mercats d'assegurança de medicaments implica que els pacients amb malalties cròniques que requereixen fàrmacs cars s'enfronten a un cost compartit elevat, cosa que essencialment reintrodueix la subscripció mèdica implícita. Això transfereix diners dels afiliats malalts als patrocinadors dels plans. Els fabricants han intentat disminuir l'efecte del cost compartit elevat mitjançant programes d'assistència, com els cupons de copagament ("copayment coupons"), on el fabricant paga el cost compartit en nom del consumidor. Aquests cupons estan permesos al mercat comercial, però no en programes governamentals com Medicare i Medicaid. La lògica estratègica per a les empreses és clara: un cupó actua com un descompte addicional, especialment amb coassegurances elevades. Les implicacions varien segons el tipus de competència; amb competència genèrica, els cupons poden desplaçar la quota de mercat cap a la versió de marca i augmentar els beneficis del fabricant original, sense augmentar necessàriament l'accés a la molècula. Per a productes sense substituts genèrics, els cupons augmenten el preu net (aproximadament un 8%) perquè debiliten la capacitat de l'assegurador comercial d'utilitzar el cost compartit en les negociacions, però augmenten l'accés a molècules específiques. Els PBMs i els patrocinadors dels plans intenten contrarestar aquestes estratègies, fins i tot excloent fàrmacs dels seus formularis. Els cupons no són rendibles per als fabricants si el fàrmac no està cobert. Limitar el cost compartit (com el límit per a la insulina a Medicare Part D sota la IRA) redueix la capacitat dels asseguradors d'utilitzar el cost compartit com a eina de negociació.
La transició de l'exclusivitat de mercat és important. Després que expiri la protecció de la propietat intel·lectual, es requereix una competència robusta. Les empreses de marca tenen incentius per estendre el seu període d'exclusivitat mitjançant l'ús extensiu de patents i secrets comercials. Els fàrmacs moderns complexos sovint estan coberts per múltiples patents (sobre la molècula, el procés de producció, nous usos), cosa que pot crear un "patent thicket" que augmenta els costos d'entrada per als competidors. Mentre que algunes firmes probablement fan servir estratègies de "thicketing", el simple fet de tenir nombroses patents no prova intencions nefastes; moltes innovacions impliquen nous usos per a productes existents, que la societat pot valorar. Les preocupacions s'haurien de centrar en la validesa de les patents subjacents.
Un sistema que funcioni bé requereix una competència robusta post-exclusivitat. Per a molècules petites, els competidors genèrics poden fabricar un producte bioequivalent exacte. L'entrada de genèrics porta a grans reduccions de preus en mercats prou grans per a múltiples entrants. Tanmateix, en mercats petits, hi pot haver espai per a pocs o només un fabricant, cosa que els pot donar poder de preu. Trobar maneres de reduir els costos i retards d'entrada per als genèrics podria augmentar els beneficis per als consumidors.
Moltes innovacions recents són productes biològics de molècula gran. En aquest cas, els nous entrants creen biosimilars, que clínicament s'assemblen al producte de referència, però no són realment bioequivalents. Els mercats de biosimilars han tingut dificultats per aconseguir reduccions de preus similars a les dels genèrics. Com que no són bioequivalents, les polítiques actuals no permeten la substitució automàtica a la farmàcia. Els pacients que ja utilitzen un producte biològic de marca poden ser reticents a canviar a un biosimilar. Els fabricants de biosimilars han de comercialitzar els seus productes de manera activa.
L'estructura dels contractes de rebaixa també pot crear barreres d'entrada per als biosimilars. Els contractes per a productes incumbents poden condicionar les rebaixes a que el PBM no inclogui productes rivals (una "rebaixa mur" o "trampa"). Un patró d'aquests contractes pot desincentivar els fabricants potencials de biosimilars.
En resum, els incentius per a la innovació farmacèutica estan fortament influenciats per les barreres als nous productes i els detalls de la propietat intel·lectual. Preguntes importants inclouen la validesa de les patents addicionals, les regles per a fàrmacs de mercats petits, les regles per a l'entrada de biosimilars, i les regles que regeixen les estructures contractuals de formularis, rebaixes i cost compartit. Les empreses incumbents busquen estendre l'exclusivitat, mentre que els entrants potencials sovint estan poc representats en l'elaboració d'aquestes regles. Confiar en l'autoregulació per obtenir una regulació òptima és temerari. Aquestes qüestions reflecteixen els compromisos generals presents en un sistema que utilitza incentius de mercat per promoure i mantenir la innovació que crea benestar.
12 de gener 2015
Involved in our own health
From King's Fund report:
Different perspectives (p.11)
The forgotten perspective in the list, though quoted in the text (p.16): A behavioural economics approach: we try to do our best for our health but the autopilot decides without our full control. Some help (nudging) is needed.• A consumerist approach: health and health care is seen as a marketplace in which patients (consumers) are involved by making choices about services, and the health care market responds to their preferences. Patient involvement is then a means to improve quality.
• A democratic approach: people have political, social and economic rights as citizens, and those who use or are affected by a public service should be involved in how it is run, and have certain rights regarding what they receive from that service.
• An ethical and outcomes-based approach: involvement is seen as the ethical thing to do, and the best approach to improve outcomes. This means recognising that good care comprises the application to individual circumstances of evidence-based medicine along with knowledge and experience. Patient involvement is essential to the judgement of relative risk and benefit associated with decision-making.
• A value-based approach: to achieve truly the best value for money from our health and care system, we must know and respond to what people need and want. In this way, we can deliver care that meets their preferences and patients receive ‘the care they need (and no less), and the care they want (and no more)’ (Mulley et al 2012).
• An approach based on sustainability: it is increasingly difficult for health systems to provide the best possible care to everyone as the prevalence of long-term conditions increases and the population ages. By involving people in managing their own health and care, and keeping well and independent, we can minimise our use of services.
• A person-centred care approach: our health and care system should be focused on its users, promoting independence and co-ordinated around people’s full needs rather than being fragmented and siloed. Patient involvement is an essential component of delivering a more person-centred service that is tailored and responsive to individual needs and values.
The message: "Embarking on an honest conversation about rights and responsibilities requires consideration of people’s motivation and the capability to engage."
PS. Nudging and the European Union, by Alberto Alemano.
PS. BIT Publication: EAST.Four simple ways to apply behavioural insights
PS. Regarding Hepatitis C treatment costs, It is good to remember this post by U. Reinhardt:
1. Is there a maximum price above which society no longer wishes to purchase added QALYs from its health system, even with the most cost-effective treatments (e.g., Point C)?2. Should that maximum price be the same for everyone, or could there be differentials – for example, a lower maximum price for patients covered by taxpayer-financed health programs (e.g., Medicaid, Tricare, the Veterans Administration health system and perhaps Medicare), a wide range of higher prices for premium-financed commercial insurance, depending on the generosity of the benefit package that the premium covers, and yet higher prices for wealthy people able to pay out of their own resources very high prices to purchases added QALYs for the family?
14 d’abril 2020
A pandemic is not a war
Key messages from the book:
To review, our greatest threats are:
1. Pathogens of pandemic potential, which essentially means influenza and the downstream effects of antimicrobial resistance.
2. Pathogens of critical regional importance, which include Ebola, coronaviruses like SARS and MERS, other viruses such as Lassa and Nipah, and Aedes-transmitted diseases such as dengue, yellow fever, and Zika.
3. Bioterrorism and dual-use research of concern (DURC), and gain-of-function research of concern (GOFRC).
4. Endemic diseases that continue to have a major impact on the world’s health, particularly among emerging nations, including malaria, tuberculosis, AIDS, viral hepatitis, childhood diarrheal diseases, and bacterial pneumonia.
Priority 1: Create a Manhattan Project–like program to secure a game-changing influenza vaccine and vaccinate the world.
Priority 2: Establish an international organization to urgently address all aspects of antimicrobial resistance.
Priority 3: Support and substantially expand the mission and scope of the Coalition for Epidemic Preparedness and Innovations (CEPI) to fast-track comprehensive public-private vaccine research, development, manufacturing, and distribution for diseases of current or potential critical regional importance.
Priority 4: Launch the Global Alliance for Control of Aedes-Transmitted Diseases (GAAD) and coordinate with the Bill & Melinda Gates Foundation’s malaria strategy, “Accelerate to Zero.
Priority 5: Fully implement the recommendations of the bipartisan report of the Blue Ribbon Study Panel on Biodefense.
Priority 6: Establish an international organization similar to the National Scientific Advisory Board for Biosecurity (NSABB) to minimize the use of DURC and GOFRC to transmit pathogens of pandemic potential
Priority 7: Recognize that TB, HIV/AIDS, malaria, and other life-threatening infectious diseases remain major global health problems
Priority 8: Anticipate climate-change effects
Priority 9: Adopt a One Health approach to human and animal diseases throughout the world.
15 de desembre 2018
Ill-prepared for the arrival of new medicines
While in the last years the number of new drugs in the market has been limited, this trend has changed and countries may expect larger bills in the next future. The OECD report explains the main challenges of pharmaceutical innovation and says:
Despite a slowdown in growth in the 2000s, pharmaceutical spending has nevertheless increased sharply in some therapeutic areas, such as oncology and certain rare diseases where many new medicines target small population groups and command high prices. While these may well address unmet needs, they often have prices that may not be justified by the health benefits they confer.
Countries may be ill-prepared for the arrival of novel medicines targeting wide population groups. In 2013, the first of a new class of very effective but expensive
drugs known as direct-acting anti-virals (DAAs) for hepatitis C created a shock due to the potential budget impact of treating all infected people. Many countries initially restricted access to the most severely affected patients, creating frustration among patients and clinicians alike. Although subsequent entries of alternative products have created competition on prices and allowed payers to expand eligibility to treatment, the initial shock highlighted the lack of readiness of payers for such events.
In some countries, sudden, large price increases for off-patent medicines have made important treatments unaffordable for patients.
Finally, innovation is lacking in certain areas of high-unmet need, such as new antimicrobials, non-vascular dementia, and some rare diseases.The report summarises different proposals and measures that would be helpful for a government that cares about citizens' welfare. Unfortunately, this is not our case.
12 de març 2014
Against patents, again
I've just finished reading a book on patents in life sciences. As you know from previous posts , I'm convinced that there is an enormous welfare loss from current patent system. If you have the opportunity to read this book, you'll finally will arrive at the same conclusion. Although it was written before the Supreme Court ruling over the Myriad case, the message is still the same: patents contrain innovation and are extremely costly to the society. The case of Hepatitis C is explained in detail. Until some patents were exhausted there was no possibility to start research. Without such patents, new succesful and (costly) treatments have arisen (and afterwards have been patented again).
An interesting interview in Forbes magazine highlights the key issues of the book. Unfortunately times go by and alternatives to patents are not taking off.
14 d’abril 2025
Pharma, big pharma (24)
Drug Wars: How Big Pharma Raises Prices and Keeps Generics Off the Market
Llibre resumit amb IA.
El llibre "Drug Wars: How Big Pharma Raises Prices and Keeps Generics Off the Market" de Robin Feldman i Evan Frondorf examina detalladament les estratègies que utilitza la indústria farmacèutica per augmentar els preus dels medicaments i evitar que els genèrics entrin al mercat.
El "Pròleg: Grans escàndols, preus més alts" introdueix la "Pharma sota foc" i assenyala que, malgrat els excessos i les preocupacions detallades al llibre, els productes farmacèutics han contribuït immensament a millorar la salut i la qualitat de vida a tot el món. Es destaca que la investigació i el desenvolupament són costosos i que els sistemes de propietat intel·lectual són essencials per incentivar la despesa i la innovació, tot i les controvèrsies sobre els preus. Es menciona Sovaldi com una cura essencialment miraculosa per a l'hepatitis C. També es descriu l'"estranya economia dels productes farmacèutics" i es proporciona un breu resum dels medicaments genèrics.
La "Introducció: El camí sinuós cap a l'entrada de genèrics" assenta les bases per entendre la complexa regulació i els incentius que regeixen l'entrada de medicaments genèrics al mercat. Es menciona la Llei Hatch-Waxman, que ofereix diversos incentius als genèrics per entrar al mercat el més ràpidament possible, creant una complexa xarxa de regulació. També es defineixen termes clau com a ingredient actiu i via d'administració. La introducció explica que la Llei Hatch-Waxman requereix que les empreses de medicaments de marca llistin totes les patents que "podrien ser raonablement reivindicades" contra un sol·licitant de genèrics, les quals es registren en un document de la FDA conegut com a "Llibre Taronja". Es detalla el procés de la certificació de "Paràgraf IV", que al·lega que la patent llistada és invàlida o no seria infringida per la sol·licitud del medicament genèric. Aquesta certificació es considera un acte "artificial" d'infracció de patent, permetent a l'empresa de marca iniciar un litigi en un termini de 45 dies.
El Capítol 1, “Generation 1.0”: The Rise and Fall of Traditional Pay-for-Delay, se centra en la pràctica del "pagament per retard", on les companyies de marca paguen a les companyies de genèrics per retardar l'entrada dels seus productes al mercat. Es descriuen els contorns bàsics del pagament per retard i com aquest actua com un "coll d'ampolla" per a l'entrada. S'aborda si el pagament per retard és realment incorrecte des del punt de vista de les farmacèutiques.
Capítol 2: “Generació 2.0”: Complicant el Pagament per Retard. Aquest capítol explora com les companyies farmacèutiques van evolucionar les seves estratègies per retardar l'entrada de medicaments genèrics després d'una major escrutini dels acords tradicionals de "pagament per retard" ("pay-for-delay"). Aquestes noves tàctiques es denominen "Generació 2.0" i es caracteritzen per acords més complexos que inclouen disposicions no monetàries, amb l'objectiu d'obscurir el valor intercanviat a canvi del retard en l'entrada dels genèrics.
- S'introdueixen exemples d'aquests acords de la Generació 2.0, com ara acords laterals ("side deals") que van més enllà dels pagaments en efectiu directes.
- Es discuteix el cas In re K-Dur, considerat generalment el primer acord de la Generació 2.0 que va enfrontar un important desafiament judicial, i com va portar a una sentència que els pagaments per retard podien abastar més que pagaments directes en efectiu.
- El capítol també analitza el cas In re Lipitor de Pfizer contra Ranbaxy, que il·lustra la complexitat d'analitzar múltiples acords en casos de la Llei Hatch-Waxman i la dificultat de definir l'abast d'un pagament per retard.
- S'introdueix el concepte de "clàusules d'escolta" ("boy scout clauses"), on les companyies de marca prometen un bon comportament, però d'una manera que té efectes anticompetitius.
- Es detalla el cas King Drug de Glaxo contra Teva, on un acord de "no genèric autoritzat" ("no-authorized-generic") es va considerar un possible "transferiment invers inusual i inexplicable de valor considerable" sota la doctrina Actavis.
- Es menciona com, després de la decisió Actavis, els acords amb disposicions no monetàries es van convertir en una manera per a les companyies de marca d'intentar eludir la creixent escrutini antimonopoli sobre els pagaments purs en efectiu per retard.
Capítol 3: “Generació 3.0”: Noves Tàctiques per a l'Obstrucció Activa de Genèrics. Aquest capítol se centra en les estratègies de la "Generació 3.0", on les companyies de marca deixen de banda la col·lusió amb els fabricants de genèrics i passen a obstaculitzar activament l'entrada d'aquests al mercat. Aquestes tàctiques utilitzen processos administratius, esquemes reguladors relacionats amb la Llei Hatch-Waxman i modificacions de medicaments.
- Es descriu l'estratègia del "salt de producte" ("product hopping"), on una companyia de marca retira del mercat la seva versió original d'un medicament i la substitueix per una versió lleugerament modificada just abans que els genèrics de la versió original puguin entrar al mercat (per exemple, el pas d'AstraZeneca de Prilosec a Nexium, el de Warner Chilcott d'Asacol a Delzicol i el d'Actavis de Namenda a Namenda XR). S'explora com aquestes modificacions sovint tenen un benefici clínic mínim o nul.
- Es torna a mencionar l'ús de clàusules d'escolta en el context del salt de producte, on una companyia de marca es compromet a no utilitzar tàctiques antagonistes després d'haver-les desenvolupat inicialment (com en el cas d'Endo i Opana ER).
- El capítol destaca la "multiplicitat de tàctiques", on les companyies combinen diverses estratègies d'obstrucció per maximitzar el retard en l'entrada de genèrics.
Capítol 4: “Generació 3.0”: Obstrucció Contínua de les Vies Reguladores. Aquest capítol continua examinant les tàctiques de la Generació 3.0, centrant-se específicament en la distorsió de les vies d'aprovació reguladores.
- Es detalla l'estratègia del retard basat en REMS (Estratègies d'Avaluació i Mitigació de Riscos), on els programes REMS requerits per la FDA per a medicaments amb riscos greus s'utilitzen presumptament per retardar l'entrada de genèrics, dificultant que aquests obtinguin les mostres necessàries per a les proves (per exemple, els casos d'Actelion i Suboxone).
- El capítol explora el retard mitjançant peticions ciutadanes, on les companyies de marca presenten peticions a la FDA sol·licitant que es retardi o s'impedeixi l'aprovació de les sol·licituds de medicaments genèrics, sovint plantejant preocupacions sobre la seguretat o la bioequivalència (per exemple, la petició de Mutual Pharmaceuticals sobre el suc de taronja i la felodipina, les peticions de Warner Chilcott sobre Doryx i les accions de Reckitt Benckiser amb Suboxone).
- Finalment, es discuteix l'estratègia per prevenir l'"etiqueta prima" ("skinny label") bloquejant les excepcions ("carve-outs"). Les companyies de marca intenten evitar que les etiquetes dels genèrics ometin usos del medicament que encara estan protegits per patents, mitjançant petits canvis en l'etiquetatge i la presentació de peticions ciutadanes (com en els casos de Skelaxin, Crestor i Abilify).
En conjunt, aquests capítols il·lustren la creixent sofisticació de les tàctiques utilitzades per les grans farmacèutiques per mantenir els preus elevats dels medicaments i evitar la competència dels genèrics, utilitzant tant acords complexos com la manipulació de vies reguladores.
El Capítol 5, Empirical Evidence of a Citizen’s Pathway Gone Astray, presenta evidències empíriques de com la via de les peticions ciutadanes s'ha desviat del seu propòsit. Es descriu la metodologia utilitzada per analitzar aquestes peticions. Es presenten els resultats de l'estudi i es discuteix el camí a seguir. Una taula mostra totes les peticions relacionades amb retards per any (2000-2012). Les figures il·lustren els mesos des de la presentació de la petició ciutadana fins a l'aprovació del genèric en diferents períodes.
La "Conclusió: Una crida a la reforma sistemàtica" examina els danys socials causats per aquestes pràctiques i proposa sistemes, simplificació, transparència i doctrines basades en estàndards com a vies per a la reforma.
Diverses tàctiques per retardar l'entrada de genèrics es detallen al llarg del llibre:
- Pagament per retard (Pay-for-Delay): Les empreses de marca arriben a acords amb les empreses de genèrics, pagant-los per retardar la introducció de versions genèriques més barates. Aquests acords poden adoptar diverses formes, inclosos els "acords laterals". El cas Actavis és mencionat en relació amb la il·legalitat potencial d'aquests pagaments enrere. El cas In re K-Dur va trobar que un acord de llicència era un "pagament enrere" per al retard genèric. El cas Teva també va implicar un pagament per resoldre una disputa per retardar el medicament per a la narcolèpsia Provigil.
- Ús estratègic de la Llei Hatch-Waxman: Les complexitats de la llei són explotades per les empreses de marca per allargar la protecció de les patents. La certificació de Paràgraf IV i el període d'exclusivitat de 180 dies per al primer genèric que desafia una patent són àrees on es produeixen moltes "guerres". La possibilitat de "parar" el període d'exclusivitat de 180 dies per part del primer sol·licitant genèric es menciona com un "coll d'ampolla".
- Product Hopping: Les companyies de marca modifiquen lleugerament un medicament (per exemple, una nova formulació o via d'administració) i intenten que els pacients canviïn al nou producte abans que el genèric del producte original pugui entrar al mercat. Exemples inclouen el canvi d'AstraZeneca de Prilosec a Nexium i el canvi d'Endo d'Opana ER a Opana ER CRF. El concepte de "boy scout clauses" es relaciona amb aquestes tàctiques.
- Abús de REMS (Risk Evaluation and Mitigation Strategies): Els programes REMS, dissenyats per garantir un ús segur de medicaments amb riscos elevats, es poden utilitzar per bloquejar o retardar l'entrada de genèrics, per exemple, negant mostres del medicament de marca necessàries per a les proves de bioequivalència. El cas d'Actelion es menciona com un exemple d'una empresa farmacèutica que es va negar a vendre mostres del seu medicament Tracleer a una empresa de genèrics. El cas In re Suboxone il·lustra com les companyies de marca poden utilitzar els REMS i les peticions ciutadanes en un intent de retardar l'entrada de genèrics.
- Peticions Ciutadanes: Les companyies de marca presenten peticions a la FDA, de vegades sense mèrit substancial, per intentar retardar l'aprovació de les sol·licituds de medicaments nous abreujades (ANDA) per a genèrics. L'anàlisi empírica del llibre mostra que aquestes peticions s'utilitzen com un esforç d'últim recurs per retardar l'entrada de genèrics. Exemples inclouen la petició de Mutual Pharmaceuticals relacionada amb el felodipina i el suc de taronja, i les peticions d'Endo relacionades amb Opana ER i de Reckitt Benckiser relacionades amb Suboxone. El cas de Skelaxin (metaxalona de King Pharmaceuticals) il·lustra com l'addició de patents de mètode d'ús i un etiquetatge intel·ligent, juntament amb peticions ciutadanes, poden conduir a anys de retard. La tàctica de prevenir l'"etiqueta magra" (skinny label) mitjançant peticions ciutadanes també es discuteix. Els casos de Crestor (AstraZeneca) i Abilify (Otsuka) mostren intents de bloquejar els genèrics mitjançant qüestions d'etiquetatge pediàtric i la Llei de Medicaments Orfes.
El llibre també assenyala la importància de la transparència i la necessitat de reformes per evitar aquestes pràctiques i garantir que els medicaments genèrics assequibles estiguin disponibles per als consumidors.
17 de juny 2025
Pharma, big pharma (34)
Rethinking Medications: Truth, Power, and the Drugs You Take
Resumit amb IA.
El Dr. Jerry Avorn, en el seu llibre "Rethinking Medications: Truth, Power, and the Drugs You Take", analitza en profunditat el recorregut dels medicaments des del seu descobriment científic fins al seu ús pels pacients, assenyalant tant els avenços impressionants de la ciència mèdica com les profundes deficiències sistèmiques que impedeixen que aquests medicaments beneficiïn plenament la societat. L'autor, metge d'atenció primària i investigador, presenta els medicaments com el "hardware" i els sistemes que en modelen l'ús (proves, patentats, preus, prescripció) com el "software", argumentant que no es pot entendre l'un sense l'altre.
El llibre s'articula al voltant de quatre eixos principals que defineixen la "cascada de decisions crucials" sobre els fàrmacs: la seva eficàcia, la seva seguretat, el seu cost i la comunicació sobre ells.
1. L'Eficàcia dels Medicaments: Com sabem si funcionen? El Dr. Avorn traça la història de la regulació de fàrmacs als Estats Units:
- Abans de 1906, qualsevol producte podia ser venut com a medicina sense revelar-ne el contingut ni provar-ne la seguretat o l'eficàcia.
- El 1938, es va exigir que els medicaments no fossin verinosos.
- El 1962 (Esmenes Kefauver), arran de la tragèdia de la talidomida, es va introduir el requisit que els fabricants havien de demostrar que els seus productes eren efectius (o més precisament, "eficaces" en l'entorn de l'assaig clínic) abans de vendre'ls. Aquesta legislació va fer de l'assaig clínic aleatoritzat i controlat (RCT) el "detector de veritat" més potent per determinar l'eficàcia d'un medicament.
Tot i això, l'autor denuncia un declivi recent en els estàndards d'eficàcia:
- Aprovació accelerada (Accelerated Approval): Aquest programa de la FDA, creat inicialment durant la crisi de la SIDA als anys 80 per agilitzar l'accés a tractaments per a malalties greus sense opcions, permet l'aprovació basada en "mesures surrogades" (resultats de laboratori o d'imatge que es "raonablement esperen" predir un benefici clínic futur) en lloc de beneficis directes per a la salut o la supervivència del pacient.
- Abús del sistema: Sota la pressió de la indústria farmacèutica i la pressió política, aquest camí s'ha expandit massa, amb empreses que sovint descuiden o retarden els estudis de seguiment confirmatoris requerits per provar el benefici clínic real. El llibre critica que molts fàrmacs aprovats així no demostren un benefici real en els estudis de seguiment o fins i tot empitjoren els pacients.
- Aduhelm (Alzheimer): Aprovat per la FDA el 2021 basant-se en una reducció de la placa amiloide al cervell (una mesura surrogada), malgrat que els assaigs clínics no mostraven un benefici clar en la funció cognitiva dels pacients i comportava riscos com la inflamació cerebral i hemorràgies.
- Leqembi (Alzheimer): Aprovat amb un "lleuger canvi en la funció cognitiva", que potser no és "clínicament significatiu" o "perceptible" per als pacients o les seves famílies, però que la FDA va aprovar perquè era "estadísticament significatiu".
- Fàrmacs per a la distròfia muscular (Exondys 51, Elevidys): Aprovats basant-se en canvis "minúsculs" en els nivells de distrofina (mesura surrogada) sense cap benefici clínic clar, amb costos anuals astronòmics ($750.000 a $1.5 milions per Exondys 51, $3.2 milions per Elevidys) i estudis de seguiment no completats o fallits.
- L'autor refuta la idea llibertària que metges i pacients poden determinar l'eficàcia dels fàrmacs, ja que les dades d'assaigs clínics són complexes, sovint secretes i subjectes a publicació selectiva per part dels fabricants.
2. La Seguretat dels Medicaments: Com detectem els riscos? Qualsevol medicament eficaç comporta riscos, i l'objectiu és quantificar-los per decidir si els beneficis valen la pena.
- Tragèdies històriques: El desastre de la sulfanilamida (1937) que va portar al requisit de proves de toxicitat (1938), i el cas de la talidomida (anys 60), que va subratllar la necessitat de proves de seguretat abans de la comercialització.
- Vioxx: Aquest analgèsic antiinflamatori va ser un cas emblemàtic. Merck, el fabricant, va interpretar o representar erròniament les dades que suggerien un augment del risc de patir atacs de cor i accidents cerebrovasculars. L'autor detalla com la companyia va intentar "enfosquir els riscos" amb "jiujitsu verbal" i "tots els esforços per silenciar els investigadors que van plantejar preocupacions". La FDA no tenia prou autoritat per exigir estudis de seguiment post-comercialització fiables.
- Vigilància post-comercialització insuficient: Abans de Vioxx, la FDA depenia principalment d'informes espontanis d'efectes secundaris, un mètode poc fiable. La crisi del Vioxx va portar a la creació del sistema Sentinel el 2007, que utilitza grans bases de dades computeritzades de l'ús de medicaments i resultats de salut de milions d'americans per detectar senyals primerencs de riscos.
- Transparència de les dades dels assaigs clínics: Escàndols com la supressió de dades d'antidepressius (Paxil) i la mala gestió de les dades d'Avandia van portar a la creació de ClinicalTrials.gov, que requereix el registre prospectiu de tots els estudis en humans. No obstant això, la divulgació completa dels resultats encara és incompleta.
3. El Cost i l'Accessibilitat dels Medicaments: Per què són tan cars? Els Estats Units tenen els preus de medicaments més alts del món. El llibre explora les causes:
- Abús del sistema de patents:
- Patents "trivials": Concessió de patents per a modificacions mínimes de fàrmacs existents (ex: versions "esquerres" de molècules com Nexium/esomeprazole) que no aporten beneficis clínics significatius, però allarguen els monopolis.
- "Thickets" de patents (Patent thickets): Acumulació de centenars de patents addicionals sobre un mateix fàrmac per estendre l'exclusivitat, com en medicaments d'alt cost com Enbrel o Humira. El 66% de les patents s'atorguen després que el medicament sigui aprovat.
- "Product hopping": Estratègies per retirar del mercat fàrmacs amb patents que expiren, forçant els pacients a canviar a versions més noves i protegides (ex: Aricept 23, Truvada/Descovy).
- Patents sobre estratègies de mitigació de risc (REMS): Patentar sistemes de seguiment de fàrmacs (ex: Xyrem) per estendre monopolis.
- Llei Bayh-Dole de 1980: Va permetre a universitats i institucions sense ànim de lucre llicenciar a empreses privades drets sobre descobriments finançats públicament (sovint amb milers de milions de dòlars via NIH). El Dr. Avorn argumenta que això ha socialitzat el risc (finançament públic) i privatitzat el guany. Exemple: Xtandi, un fàrmac contra el càncer de pròstata desenvolupat amb fons públics, es ven als EUA a un preu fins a sis vegades superior que a altres països. Sovaldi (hepatitis C) és un altre exemple: costava $1.000 la píndola ($84.000 el tractament) amb gairebé cap despesa de recerca per part de Gilead.
- Llei de Medicaments de Medicare de 2003: Aquesta llei va prohibir explícitament a Medicare (el major programa de salut federal) negociar els preus dels medicaments, fent-los l'únic producte per al qual el govern no pot negociar els costos.
- Gestors de Beneficis Farmacèutics (PBMs): Aquests intermediaris, sovint part de corporacions verticalment integrades, negocien preus amb els fabricants. El llibre argumenta que molts dels descomptes (rebates) que obtenen els PBMs no es transfereixen als pacients o asseguradores, i paradoxalment, incentiven l'ús de fàrmacs més cars dels quals reben majors comissions.
- Preus astronòmics de nous fàrmacs: Casos com els medicaments GLP-1 (Ozempic, Wegovy), que tot i ser efectius i segurs, costen al voltant de $1.000 al mes i impedeixen l'accés a molts.
- El cost-eficàcia (QALY): El llibre aborda la idea de calcular el cost per any de vida ajustat per qualitat (QALY) com una manera de determinar un preu just, però aquesta pràctica és il·legal als EUA.
- Lobbying de la indústria: La indústria farmacèutica gasta més en lobbying que qualsevol altre sector, influint en decisions polítiques que mantenen els preus elevats.
4. La Comunicació sobre Medicaments: Qui ens informa? La informació sobre medicaments no és neutral i està fortament influenciada pel màrqueting.
- Màrqueting dominant: La indústria farmacèutica gasta més de $35 mil milions a l'any en promoció, influint en el coneixement de metges i pacients.
- Promoció "off-label" i llibertat d'expressió: Casos judicials com el de Caronia (Xyrem) han qüestionat l'autoritat de la FDA per regular les afirmacions promocionals dels fabricants, suggerint que la promoció "off-label" (per a condicions no aprovades) podria estar protegida per la llibertat d'expressió. Això podria conduir a un "estat de naturalesa" farmacèutic, on la informació no estaria filtrada per l'evidència.
- Educació mèdica: El llibre critica la insuficient formació dels metges en l'avaluació crítica de medicaments i la influència de la indústria en els plans d'estudis mèdics.
- "Academic Detailing" (Alosa Health): El Dr. Avorn va desenvolupar aquest enfocament d'educació proactiva i basada en l'evidència per a professionals de la salut, utilitzant principis eficaços de canvi de comportament per promoure una prescripció òptima i independent de la influència comercial. Aquest programa ha demostrat millorar la prescripció i estalviar diners.
Casos d'Estudi Il·lustratius:
- La crisi dels opioides: Descriu com la FDA va asseure les bases per a l'epidèmia en aprovar l'ús d'opioides per al dolor crònic sense assaigs a llarg termini que demostressin la seva eficàcia i seguretat a llarg termini. El llibre destaca la influència de fabricants com Purdue Pharma (OxyContin) i Insys (Subsys) que van enganyar metges i reguladors sobre l'addictivitat i van promoure l'ús "off-label".
- Psicodèlics (MDMA per a PTSD): S'analitza el potencial terapèutic dels psicodèlics, però també les dificultats de la FDA per regular un tractament que combina una molècula amb una teràpia especialitzada ("set and setting"). El llibre detalla els problemes amb els assajos clínics de MAPS/Lykos per MDMA per PTSD, incloent "desemmascarament funcional" i preocupacions sobre la "validesa dels resultats" que van portar a la FDA a rebutjar l'aprovació.
Solucions Proposades ("Rethinking"): El Dr. Avorn ofereix solucions pràctiques per a cada problema:
- Eficàcia: Limitar l'aprovació accelerada, implementar aprovacions condicionals amb seguiment rigorós, fomentar estudis clínics pragmàtics finançats públicament i crear organitzacions independents d'avaluació de tecnologia sanitària (HTA) com l'ICER.
- Seguretat: Enfortir l'autoritat de la FDA per a la vigilància post-comercialització (llei FDORA), millorar la transparència de les dades dels assaigs clínics (ClinicalTrials.gov) i assegurar que els resultats negatius també es publiquin.
- Cost: Reformar el sistema de patents (criteris més estrictes, facilitar la impugnació de patents dubtoses, podar "thickets"). Fer complir correctament la Llei Bayh-Dole (termes raonables, drets de "march-in"). Permetre a Medicare negociar els preus dels medicaments. Implementar anàlisis de cost-eficàcia basats en el valor clínic real. Recolzar el desenvolupament de medicaments sense ànim de lucre (Civica Rx, IGI, Odylia Therapeutics).
- Comunicació i Educació: Redirigir els fons de màrqueting de la indústria cap a la generació i difusió d'informació imparcial sobre medicaments. Millorar l'educació mèdica sobre l'avaluació de l'evidència i la política farmacèutica. Promoure el "detallat acadèmic" per a una prescripció basada en l'evidència.
En resum, el llibre és una crítica exhaustiva al sistema actual de medicaments als EUA, que argumenta que la lògica de "maximitzar els beneficis" de les corporacions, unida a la inèrcia o la influència política en les agències reguladores i les institucions acadèmiques, ha creat un sistema disfuncional. Malgrat la foscor d'aquests problemes, el Dr. Avorn conclou amb un missatge d'optimisme, afirmant que aquests reptes són "abordables" i que les solucions pràctiques existeixen si hi ha voluntat de donar prioritat a la salut del pacient per sobre dels interessos comercials. El seu objectiu final és capacitar pacients i professionals per prendre un paper més actiu en la comprensió i la configuració del futur dels medicaments.
PS. En Javier Padilla sobre la transparència de preus dels medicaments
03 de novembre 2024
La gestió de la innovació a les ciències de la vida
Managing Discovery in the Life Sciences
Aquest llibre explora els factors clau que impulsen la invenció en les ciències biomèdiques, centrant-se en com els descobriments es transformen en productes comercialitzables.
El llibre s'estructura al voltant de tres preguntes fonamentals: Quins incentius o recompenses van impulsar el descobriment? D'on provenien els recursos per a la investigació i el descobridor? Com es va traduir el descobriment en un producte comercialitzable?
El llibre analitza diversos casos d'estudi que mostren els diversos camins i graus d'èxit en el procés de descobriment i comercialització.
S'hi inclouen exemples com els estatines (inhibidors de la síntesi del colesterol), els inhibidors de l'ECA (utilitzats per tractar la hipertensió), l'angioplàstia (un procediment per obrir artèries bloquejades), el tractament de l'hepatitis C, la recerca de tractaments per a la malaltia d'Alzheimer [2] i la història de la metformina (un medicament per a la diabetis).
El llibre també examina la importància de l'estructura organitzativa per fomentar descobriments comercialitzables. Analitza com els incentius, l'assignació de recursos i la creació de cultures innovadores influeixen en la productivitat i la creativitat en la recerca.
Finalment, el llibre es pregunta si les polítiques públiques podrien millorar el procés de gestió dels descobriments biomèdics per beneficiar la societat.
A més a més, el llibre busca desmentir les crítiques infundades dirigides a la indústria biomèdica, especialment a la indústria farmacèutica, posant de manifest els beneficis que ha aportat a la societat a través dels seus descobriments i medicaments.
El llibre conclou amb una crida a la reflexió sobre com podem augmentar el flux de nous productes sense incrementar els costos de R+D. S'interroga sobre la millor manera d'assignar els fons de recerca bàsica i de traduir els descobriments en productes comercialitzables de forma més ràpida i econòmica.
21 de novembre 2011
Longevitat i despesa
Un cop d'ull a una dècada sencera ens ofereix una resposta: altres països ho han fet millor. En una dècada hem guanyat 2,5 anys de vida tot creixent al 4,5 % anual la despesa sanitària. Algú dirà, per aconseguir salut no tot ha de ser amb serveis sanitaris, cert, ho sé. Un altre dirà, parles d'anys de vida i interessen els anys de vida sense discapacitat. Admetent un cert reduccionisme, el cert és que tenim el gràfic adjunt a Society at a Glance, informe de l'OCDE:
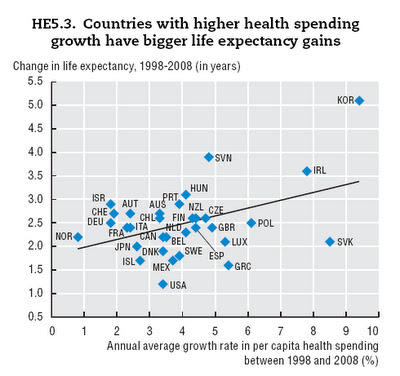
Ja ho veieu, pocs ens superen per la cua (gasten més i assoleixen menys o igual nombre d'anys). Hi trobem UK, Polònia, Txèquia, Eslovàquia, Grècia i Luxemburg. En cap cas crec que el títol del gràfic sigui el més encertat, i la relació causal que s'endevina, més despesa major longevitat, resta lluny de ser explicada acuradament.
Ara que s'acosta l'aprovació de pressupostos i veient això, hi ha motius per a pensar en termes marginals: sabem què n'obtenim a canvi per cada euro addicional gastat en sanitat?
PS. I jo avui em pregunto també, algú sap quants diners hem gastat en bevacizumab per càncer de mama? Ho dic perquè el cost incremental respecte taxans és de 37.209€ per tractament i ara, just aquest divendres la FDA ha retirat la indicació, són diners llençats. Si multipliquem aquesta xifra pel nombre de tractaments representa un cost d'oportunitat notable per al sistema de salut, ens ho recompensarà EMA i FDA?. Recordeu allò de la impunitat del regulador? Ja en teniu un altre exemple..
PS. Circle és realment la solució màgica als problemes del NHS? Per conèixer-ho consulteu FT
PS. El dia després de les eleccions convé reflexionar encara una mica més que el dia abans. En especial cal que ho facin els que van impulsar una campanya electoral contaminada premeditadament. Els que van ser objecte del meu comentari fa uns dies al blog ja poden reflexionar pausadament sobre el daltabaix, el que va provocar aleshores l'anunci, i els resultats que n'han rebut després. Evidentment carregar-ho tot a un anunci és un despropòsit, però l'anunci cal prendre'l ara en termes de metàfora-boomerang metge-diputat.
PS. L'article de Habermas a FAZ el trobareu resumit a The Guardian.
PS. El que ens trobarem ben aviat, nou medicament per Hepatitis C..








