China Rx_ Exposing the Risks of America’s Dependence on China for Medicine
09 d’agost 2019
08 d’agost 2019
07 d’agost 2019
06 d’agost 2019
05 d’agost 2019
04 d’agost 2019
03 d’agost 2019
02 d’agost 2019
01 d’agost 2019
31 de juliol 2019
Costing genome sequencing
The complete costs of genome sequencing: a microcosting study in cancer and rare diseases from a single center in the United Kingdom
The aspirational cost of sequencing a genome is $1000, but there is little evidence to support this estimate.How much does it cost really?
Genome sequencing costs £6841 per cancer case (comprising matched tumor and germline samples) and £7050 per rare disease case (three samples). The consumables used during sequencing are the most expensive component of testing (68–72% of the total cost).Check all the details in this article . Data related to 2017. Genome cost: £3420
30 de juliol 2019
25 de juliol 2019
Lab tests and the value of heterogeneity and stratified decision making
Establishing the Value of Diagnostic and Prognostic Tests in Health Technology Assessment.
This paper lays out a coherent framework for the assessment of diagnostic and prognostic tests for HTA using a linked-evidence, or decision modelling, approach. It is solidly grounded on the indirect mechanism of value accrual for these health technologies that can be summarised using three interlinked components: classification (using test results to define treatment groups), choice (in terms of treatment) and outcomes. Importantly, this paper proposes a series of innovative graphical displays
aiming to better inform decision making.
The literature on the value of heterogeneity and stratified decision making directly relates to this mechanism of value accrual with diagnostic and prognostic tests. Heterogeneity is defined as the variation in outcome of a population (variability) that can at least partly be explained by some attribute of interest. Heterogeneity is valuable insofar as it allows treatment decisions to be stratified across different subgroups so as to generate gains in (net)health; but, for heterogeneity to be identified, tests need to be applied that identify the subgroup an individual patient belongs to.A reference article on the topic, for the files.
Caravan Palace
20 de juliol 2019
Living drugs
The Promise and Price of Cellular Therapies
The op-ed of The New Yorker provides a clear understanding of the development of CAR-T therapies and the birth of a new class of drugs: the "living drugs". Their implications are huge, from manufacturing to pricing. A must read or listen.
The op-ed of The New Yorker provides a clear understanding of the development of CAR-T therapies and the birth of a new class of drugs: the "living drugs". Their implications are huge, from manufacturing to pricing. A must read or listen.
12 de juliol 2019
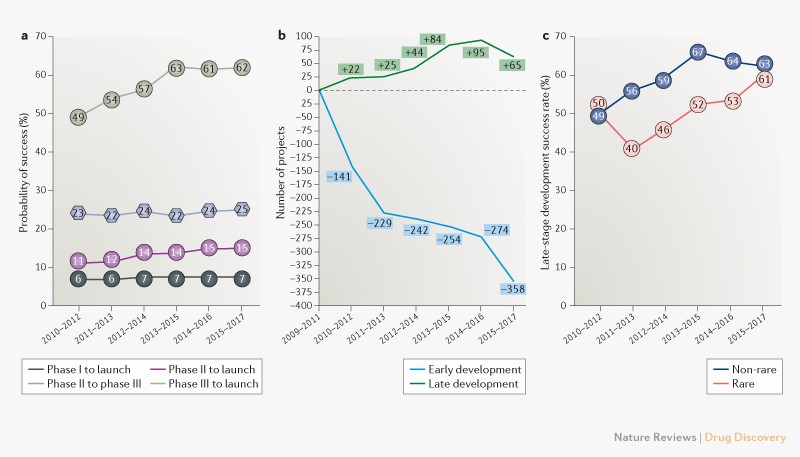
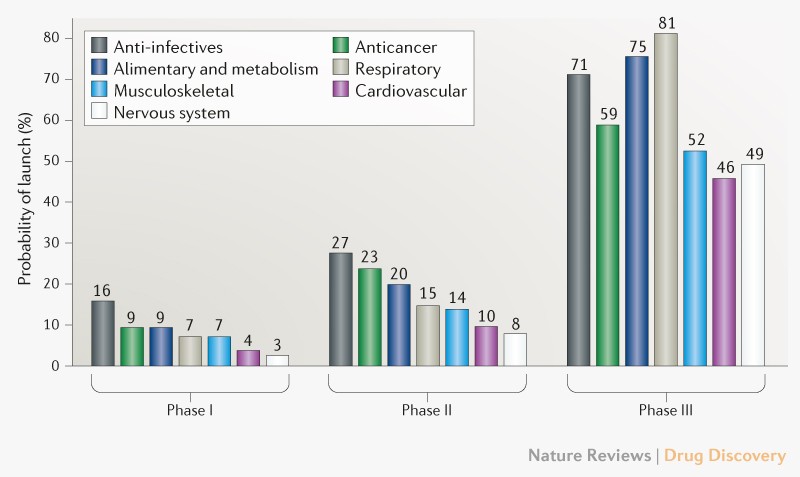
Drug development failure rate
Trends in clinical success rates and therapeutic focus
Clinical failure rates from phase I to drug launch remain at 93%.
If we look at nervous system it is the highest 97%, while the lowest failure is in anti-infectives 84%.
Clinical failure rates from phase I to drug launch remain at 93%.
If we look at nervous system it is the highest 97%, while the lowest failure is in anti-infectives 84%.
Subscriure's a:
Missatges (Atom)