En Joan Font de Comediants va decidir celebrar els 50 anys del grup fent una obra amb el títol "El venedor de fum", una història personal reflectint tot el que ha estat la trajectòria. És evident que en Joan Font és un venedor d'històries magnífiques que ens han omplert moltes hores de joia i distracció. Unes històries on l'audiència gaudia d'allò més bé. I si hagués de triar una obra de les moltes que van fer, jo diria "El llibre de les bèsties", l'obra magna de Ramon Llull posada en escena muda. Impressionant. Voldria tornar a veure-la.
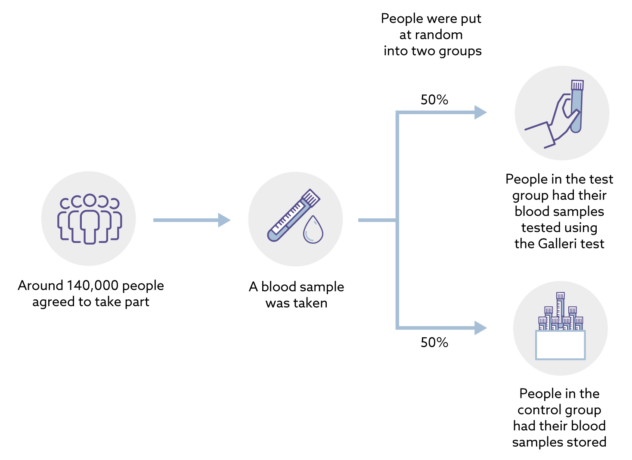
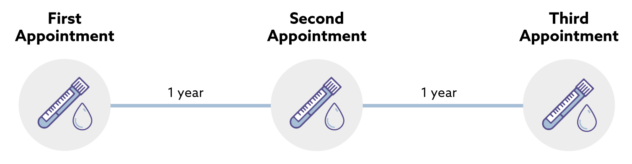
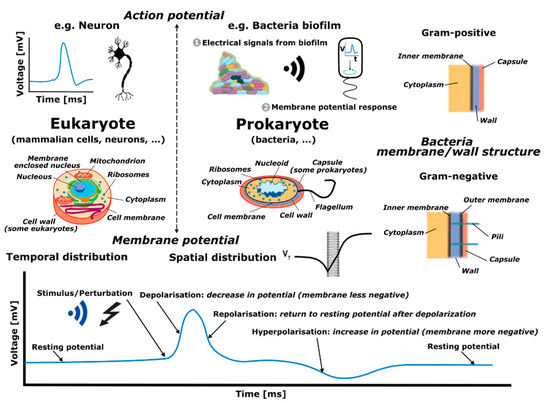
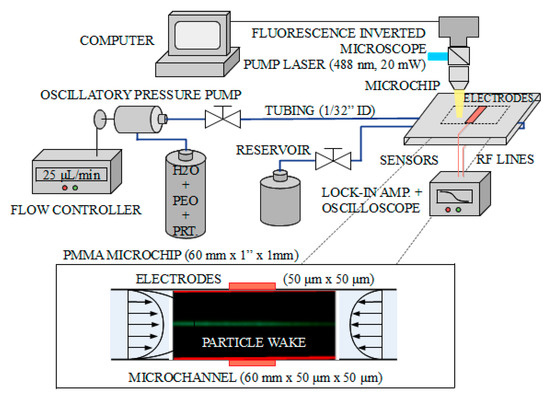
Doncs bé, de venedors de fum amb altres consequències més preocupants en tenim molts. Ahir entrava a la presó per 11 anys Elizabeth Holmes pel cas Theranos, un gran frau diagnòstic basat a Silicon Valley. N'hem parlat molt aquí. Però hi ha altres venedors de fum que sota una aureola de certesa, seguretat i eficàcia, ofereixen una profunda incertesa que sovint no acaba qualificada pels tribunals com a frau. Això succeeix quan el regulador no ha fet bé la seva feina (i els tribunals tampoc) i ja ho vaig explicar pel cas dels diagnòstics in vitro fa 6 anys. Doncs bé ara que ja és efectiva la regulació ja podem veure el desgavell que es produeix. En temps de pandèmia es va fer evident. Resulta que de les noves proves per detecció de Sars-COV-2 no en sabíem a Europa la sensibilitat i especificitat, arribaven amb una marca CE que fan uns organismes i que no serveix per saber la magnitud dels falsos positius i negatius. La solució era esperar que la FDA digués quina era la sensibilitat i especificitat d'una prova per tal de comprar-la. Tot això, en general, la gent del carrer ho desconeixia i ho desconeix, només intuïa que hi havia resultats que no eren certs i que si els feien dues vegades no sortia el mateix.
Ara que ja ha passat la pandèmia, tenim el mateix problema però agreujat. Les empreses han après que la regulació de pa sucat amb oli europea és una oportunitat a explotar. I dic això perquè veig una febre de comercialització de noves proves diagnòstiques sense cap avaluació independent i que quan hi ha una dada de sensibilitat i especificitat resulta que només és publicada principalment pels empleats o accionistes de l'empresa que les fabrica. I ara volen que els mateixos que han subvencionat la recerca siguin els seus clients. I ja hem tancat el cercle. I si voleu hi podem afegir la caixa negra de les patents i ja ho tenim tot. Just avui que hi ha tres països que passen de llarg de la nova regulació europea de patents, sabeu quins són? Democràcies plenes i modernes: Polònia, Croàcia i Espanya. I si voleu podem afegir que han patentat privadament les proves diagnòstiques quan han disposat de finançament públic. I aquí m'aturo. L'últim que tanqui el llum i la porta.