The Genome Odyssey. Medical Mysteries and the Incredible Quest to Solve Them
23 de febrer 2022
16 de maig 2020
15 de setembre 2023
Quant estem disposats a pagar pels tractaments genètics?
Gene Therapies for Sickle Cell Disease. Evidence Report
L'anèmia de cèl·lules falciformes és una malaltia genètica que afecta l'hemoglobina. És causada per l'hemoglobina S, la qual es crea al canviar una glutamina per una valina en les cadenes polipèptiques beta; això provoca que en una situació de baixa tensió d'oxigen, l'hemoglobina es deforma i l'eritròcit adquireix una aparença d'una falç. Aquesta nova forma provoca dificultats pel que fa a la circulació dels glòbuls vermells, s'obstrueixen els vasos sanguinis i causen símptomes com, per exemple, dolor en les extremitats. Els glòbuls vermells també tenen una vida més curta i, a la vegada, això provoca una anèmia, ja que no poden ser reemplaçats a temps (Wikipedia).
L’única cura per malaltia de cèl·lules falciformes és el trasplantament de medul·la òssia o de cèl·lules mare. Com que aquests trasplantaments són arriscats i poden tenir efectes secundaris greus, en general només es fan servir en nens amb malaltia de cèl·lules falciformes greu. Hi ha tractaments que poden ajudar a alleujar els símptomes, disminuir les complicacions i allargar la vida, però res definitiu per ara com un tractament genètic.
Hi ha dos tractaments genètics en desenvolupament i pendents de propera aprovació a la FDA, lovo-cel i exa-cel. Aquest darrer basat en CRISPR. Miro una anàlisi d'efectivitat comparada i costos per QALY i veig que ens situem en 193.000$ de cost per QALY amb el supòsit d'un cost d'adquisició de 2.000.000 $, tant per lovo-cel, com per exo-cel.
El debat sobre els llindars dels QALYs és de final incert. Si ens situem a 30.000€ per QALY, hauriem d'acceptar més de 6 vegades el llindar pel cas d'exa-cel. Si tenim present els costos mitjans sanitaris de la vida, el nostre article de fa 10 anys deia: 111.936€ dones, 81.566€ homes (per cert, convé actualitzar-lo). És a dir que pagaríem per any ajustat per qualitat dues vegades el cost sanitari mitjà de tota la vida. Aquesta és una mètrica que crec interessant de considerar en el debat sobre els llindars. Però malauradament ningú hi pensa per ara.
Crec que convé reflexionar seriosament sobre tot el que ve, ho tenim a prop i no podrem dir que ens ha agafat per sorpresa. Perquè altrament ja sabem el que passarà, aplicarem la regla de rescat i qui dia passa any empeny, que això ho sabem fer molt bé.
28 de maig 2020
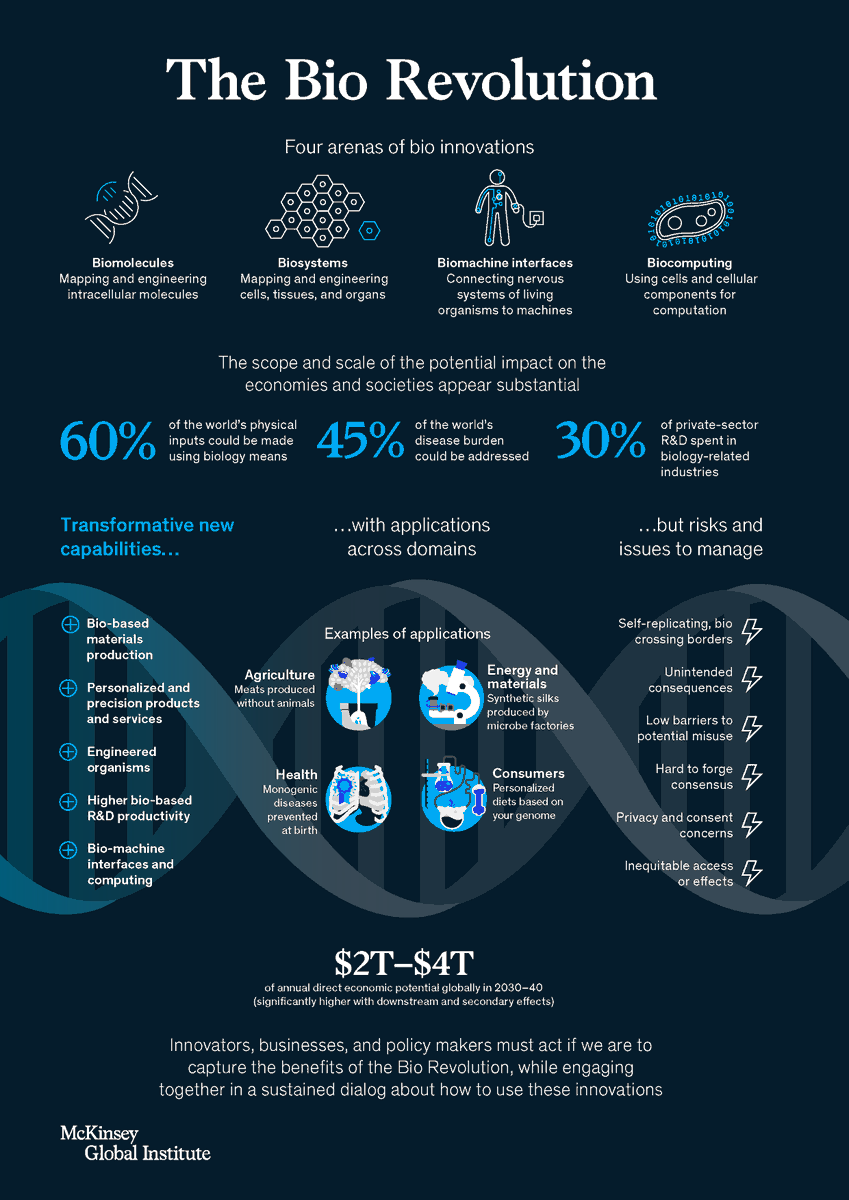
How advances in biological science are transforming economies and societies
McKinsey Global Institute is well known by their excellent papers and analysis. Forget consultancy for a while, if you are interested in the disruptive knowledge, go to MGI. Recently they have released an excellent report on the Bio Revolution. This is a timely contribution for an issue that those that read this blog already know: we are within a huge change on how life has been considered. CRISPR technology, among others, are changing quickly the landscape.
The potential scope and scale of the (direct and indirect) impact of biological innovations appear very substantial. As much as 60 percent of the physical inputs to the global economy could be produced biologically. Around one-third of these inputs are biological materials (such as wood). The remaining two-thirds are not biological materials, but could, in principle, be produced using innovative biological processes (for instance, bioplastics).
A pipeline of about 400 use cases, almost all scientifically feasible today, is already visible. These applications alone could have direct economic impact of up to $4 trillion a year over the next ten to 20 years. More than half of this direct impact could be outside human health in domains such as agriculture and food, consumer products and services, and materials and energy production. Taking into account potential knock-on effects, new applications yet to emerge, and additional scientific breakthroughs, the full potential could be far larger.A must read.
12 de desembre 2023
Quin hauria de ser el preu adient dels medicaments?
The Right Price: A Value-Based Prescription for Drug Costs
Ara que la FDA acaba d'aprovar la primera teràpia d'edició genètica mitjançant CRISPR per anèmia de cèl·lules falciformes, ja sabem el preu que ha decidit VERTEX, l'empresa comercialitzadora, 2,2 milions $. A Catalunya poden haver-hi unes 240 persones afectades i per tant l'impacte pressupostari del cost del tractament seria de més de 500 milions (si el preu final fos el que diuen ara). Aquesta és la dimensió del problema que s'acosta. La EMA l'aprovarà el mes de març proper.
Quin hauria de ser el preu adient d'un medicament?. Bona pregunta per un moment com aquest. Aquesta és precisament la qüestió que tracta un llibre de Peter Neumann et al. que he llegit recentment. Es tracta d'una obra per a tots els públics centrada als USA però que també va més enllà. L'índex ja dona una idea:
PART I. THE ECONOMICS OF PRESCRIPTION DRUGS
1.Introduction
2.The Prescription Drug Market
3.Proposed Solutions for Rising Drug Prices
4.Measuring the Value of Prescription Drugs
PART II. EXPERIENCES MEASURING A DRUG’S VALUE IN THE US AND ABROAD
5.Measuring Drug Value: Whose Job Is It Anyway?
6.Institute for Clinical and Economic Review
7.Other US Value Assessment Frameworks
8.Do Drugs for Special Populations Warrant Higher Prices?
PART III. GETTING TO VALUE-BASED PRICING FOR DRUGS
9.Improving Value Measurement
10.Aligning Prices With Value
11.The Path Forward
La tercera part m'ha interessat especialment perquè planteja qüestions sobre els QALYs que repetidament he explicat en aquest blog, i diu:
If QALYs were a person, they might receive a lot of hate mail. People complain that QALYs are not patient-focused, that they are used as rationing tools by health insurers, and that putting numbers on people’s health is dehumanizing. “The entire superstructure of the QALY methodology is built upon philosophical sand,” wrote one critic in 2019. As we have seen, the use of cost-per-QALY ratios by payers to inform drug coverage and pricing decisions attracts intense opposition in some quarters.
El racionament existeix però hipòcritament ningú en vol parlar, diu. I els QALYs tenen problemes però,
A philosopher and ethicist, Peter Singer, has observed (with apologies to Winston Churchill), QALYs may be the worst way to measure health, except for all of the others.
I així és. Som davant d'un llibre d'interès, especialment per a reguladors acabats d'arribar al càrrec i no tenen temps de llegir i s'enfronten al pànic escènic. És un llibre relativament curt que ajudarà a posar les idees en el context acurat.
PS. Tinc la impressió que cal superar la lògica del preu i anar cap una concepció diferent de contracte públic de subministrament de teràpies. Ho he explicat en altres ocasions i en canvi aquest llibre no ho reflecteix.
PS. Tinc la impressió que estem davant d'una teràpia amb gran potencial d'efecte crida, molt preocupant. Encara som a temps per regular-ho i evitar que passi com amb els peruans i el CAR-T. O es fa abans o ja serà massa tard i es farà malament.
PS. Aquí teniu un exemple de recerca en un àmbit on ja s'ha trobat la solució, i per tant és inapropiat invertir-hi. Ara bé, algú no se n'ha volgut adonar i uns altres hi estan abocant diners. Trobo a faltar informes sobre recerca fútil. Si voleu conèixer com s'ha arribat a la solució, ho trobareu aquí.
PS. Antic post sobre guerra injusta, per rellegir ara mateix.
16 d’octubre 2019
Gen-ethics (2)
The CRISPR journal has released a special issue on ethics. You'll find an article with the title "Heritable Genome Editing and the Downsides of a Global Moratorium". This is exactly the opposite argument that Françoise Baylis provides in her book. I think that it is unacceptable a recommendation that nations should "regulate HGE for safety and efficacy only and without distinguishing between therapeutic and enhancing modifications". We can't leave such decisions to scientists and practitioners. I'm really concerned about it.
23 de març 2017
Anticipating public concern over genome editing
The Nuffield Council has released a key document on ethical implications of genome editing. You'll notice that it is an open document, a work in progress because technology is evolving. If you want an excerpt check this short guide.
It should be remembered that most prospective technologies fail, and that some lead to undesirable consequences, a fact often obscured by ‘whig’ histories that reconstruct the history of successful technologies and their beneficial social consequences. Scientific discovery and technological innovation is important but not inevitable. Most important among the factors shaping technological development is human agency. It is human agency, in terms of decisions that are made about directions of research, funding and investment, the setting of legal limits and regulatory principles, the design of institutions and programmes, and the desire for or acceptance of different possible states of affairs, that will determine whether, and which, prospective technologies emerge and, ultimately,Nuffield council work is of interest, meanwhile, China is already testing CRISPR technology in humans, no ethical concerns...
their historical significance.
10 d’octubre 2019
Gen-ethics
CRISPR and the Ethics of Human Genome Editing
Our future belongs to all of us. Or maybe not? Maybe somebody could decide for us without noticing it?. The "decisions about the use of genetic technologies in humans are too important to be left to scientists", society has to achieve a broad consensus. Françoise Baylis in her new book sheds light on this issue. She explained his position in an article last spring and now she has published a remarkable book on ethics and genome editing. Her calls for an open and comprehensive international process to reach political, scientific, and social/ethical consensus on regulation of human genome editing have been unattended up to now. Her ultimate goal:
I want to live in a world that promotes equity and justice and celebrates difference, a world where everyone matters. I want to live in a world where we embrace neighborliness, reciprocity, social solidarity, and community in pursuit of human flourishing and the common good. I want to live in a world where we value collegial as opposed to competitive relations. I don’t want to live in a world where a select, privileged few are able to inscribe their privilege in their DNA and thereby exacerbate unfair class divisions and other social injustices. For these reasons, I want for all of us to reflect on whether heritable human genome editing is a boon or a threat.I agree. An essential reading highly recommended.
09 de novembre 2020
08 de juny 2020
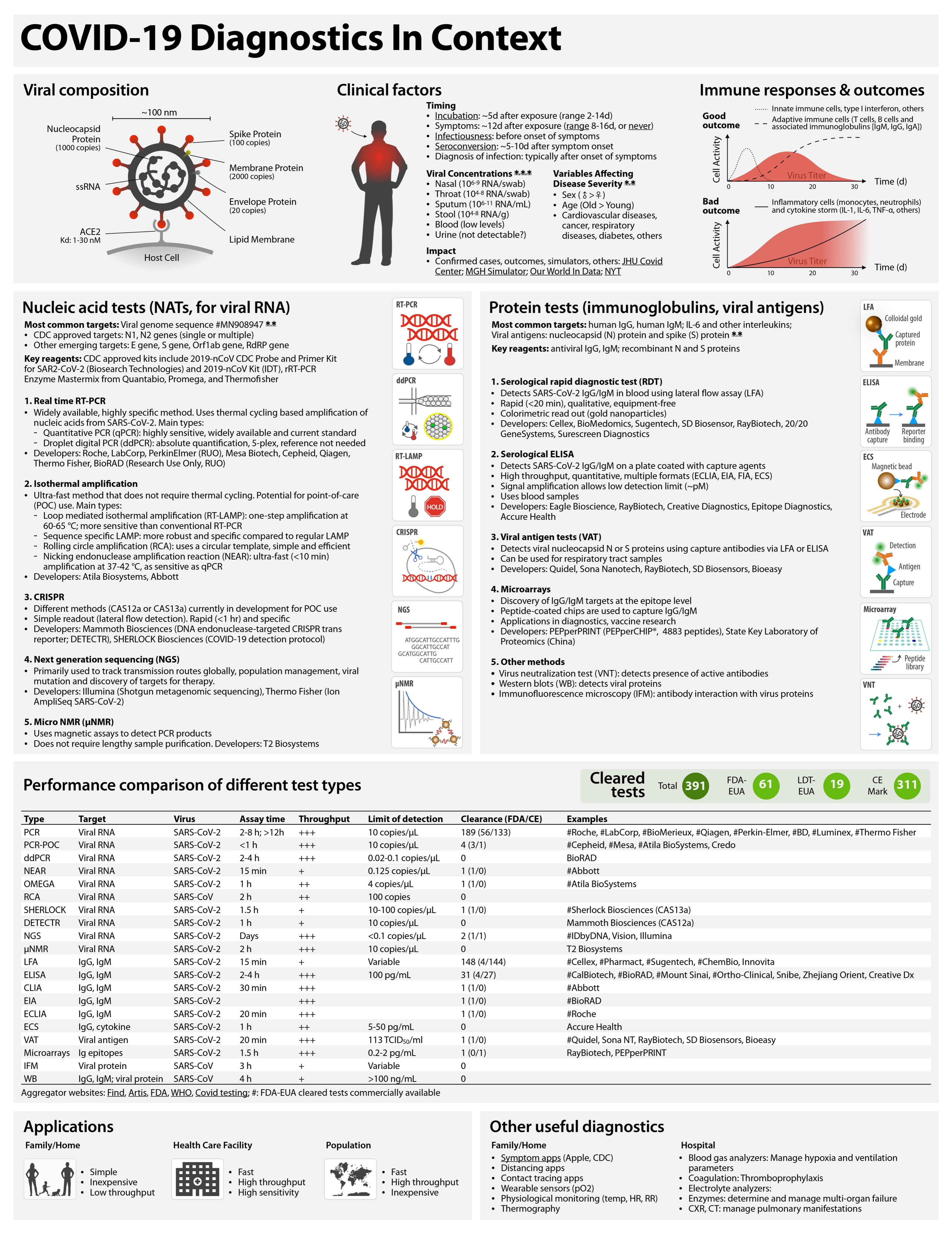
Covid-19 testing landscape
This is the best summary of current supply of diagnostic tests for Covid-19:
COVID-19 tests can be grouped as nucleic acid, serological, antigen, and ancillary tests, all of which play distinct roles in hospital, point-of-care, or large-scale population testing.Eric Topol says:
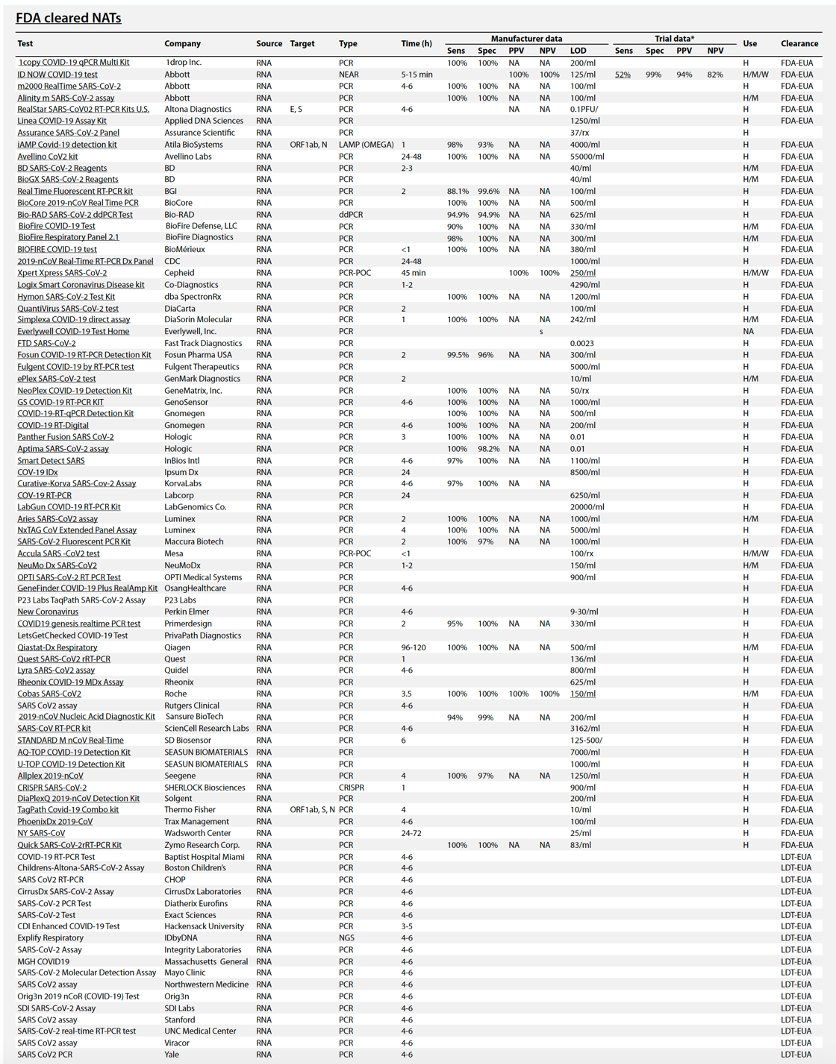
Table 1 summarizes the existing and emerging tests, current at the time of writing (May 2020). A continuously updated version of this table is available at https://csb.mgh.harvard.edu/covid
There are now *88* @US_FDA cleared (by EUA) #COVID19 tests so far. Their false negative rates range from 10-48% (by post-release reports).I agree.
Might be better to have less tests, more accuracy, with faster turnaround
Table 1 Performance comparison of different test types.
Throughput is determined by process type and assay time. In general, automated plate-based assays have higher daily throughputs. Hashtag (#) indicates example systems that have received FDA emergency use authorization (FDA-EUA). See https://csb.mgh.harvard.edu/covid to access continuously updated information. PCR, polymerase chain reaction; PCR-POC, PCR–point-of-care; ddPCR, digital droplet PCR; NEAR, nicking endonuclease amplification reaction; RCA, rolling circle amplification; SHERLOCK, specific high-sensitivity enzymatic reporter; DETECTR, DNA endonuclease-targeted CRISPR transreporter; NGS, next-generation sequencing; μNMR, micro–nuclear magnetic resonance; LFA, lateral flow assay; ELISA, enzyme-linked immunosorbent assay; CLIA, chemiluminescence immunoassay; EIA, enzyme immunoassay; ECLIA, electrochemiluminescence immunoassay; ECS, electrochemical sensing; VAT, viral antigen assay; IFM, immunofluorescence microscopy; WB, Western blot.
| Type | Target | Virus | Assay time | Process type | FDA-EUA | Examples |
| PCR | Viral RNA | SARS-CoV-2 | 2–8 hours; >12 hours | Plate | 56 | #Roche, #LabCorp, #BioMerieux, #Qiagen, #Perkin-Elmer, #Becton Dickinson, #Luminex, #Thermo Fisher, others |
| PCR-POC | Viral RNA | SARS-CoV-2 | <1 hour="" td=""> | Cartridge | 2 | #Cepheid, #Mesa, Credo |
| ddPCR | Viral RNA | SARS-CoV-2 | 2–4 hours | Manual | 1 | #BioRAD |
| NEAR | Viral RNA | SARS-CoV-2 | 15 min | Cartridge | 1 | #Abbott |
| OMEGA | Viral RNA | SARS-CoV-2 | 1 hour | Plate | 1 | #Atila BioSystems |
| RCA | Viral RNA | SARS-CoV | 2 hours | 0 | ||
| SHERLOCK | Viral RNA | SARS-CoV-2 | 1.5 hours | Kit | 1 | #Sherlock Biosciences (CAS13a) |
| DETECTR | Viral RNA | SARS-CoV-2 | 1 hour | Kit | 0 | Mammoth Biosciences (CAS12a) |
| NGS | Viral RNA | SARS-CoV-2 | Days | 1 | #IDbyDNA, Vision, Illumina | |
| μNMR | Viral RNA | SARS-CoV-2 | 2 hours | Cartridge | 0 | T2 Biosystems |
| LFA | IgG, IgM | SARS-CoV-2 | 15 min | Cartridge | 3 | #Cellex, #Sugentech, #ChemBio, Innovita |
| ELISA | IgG, IgM | SARS-CoV-2 | 2–4 hours | Plate | 4 | #Mount Sinai, #Ortho-Clinical (2), #EUROIMMUN US Inc., BioRAD, Snibe, Zhejiang orient, Creative Dx |
| CLIA | IgG, IgM | SARS-CoV-2 | 30 min | Cartridge | 2 | #Abbott, #DiaSorin |
| EIA | IgG, IgM | SARS-CoV-2 | 2 hours | Plate | 1 | #BioRAD |
| MIA | IgG, IgM | SARS-CoV-2 | Plate | 1 | #Wadsworth Center | |
| ECLIA | IgG, IgM | SARS-CoV-2 | 20 min | Plate | 1 | #Roche |
| ECS | IgG, cytokine | SARS-CoV-2 | 1 hour | Cartridge | 0 | Accure Health |
| VAT | Viral antigen | SARS-CoV-2 | 20 min | Cartridge | 1 | #Quidel, Sona NT, RayBiotech, SD Biosensors, Bioeasy |
| Microarrays | Ig epitopes | SARS-CoV-2 | 1.5 hours | Plate | 0 | RayBiotech, PEPperPRINT |
| IFM | Viral protein | SARS-CoV | 3 hours | Manual | 0 | |
| WB | IgG, IgM; viral protein | SARS-CoV | 4 hours | Manual | 0 |
11 de juny 2020
09 de desembre 2018
Claiming for global regulation of genome editing
The Nuffield Council of Bioethics release last July a key document on Bioethics of Genetics. Now that a chinese "scientist" claims to have edited the genomes of twin baby girls is the right moment to read it. And the key principles are:
Principle 1: The welfare of the future person
Gametes or embryos that have been subject to genome editing procedures (or that are derived from cells that have been subject to such procedures) should be used only where the procedure is carried out in a manner and for a purpose that is intended to secure the welfare of and is consistent with the welfare of a person who may be born as a consequence of treatment using those cells.
Principle 2: Social justice and solidarity
The use of gametes or embryos that have been subject to genome editing procedures (or that are derived from cells that have been subject to such procedures) should be permitted only in circumstances in which it cannot reasonably be expected to produce or exacerbate social division or the unmitigated marginalisation or disadvantage of groups within society.New concerns are arising from CRISPR application and international regulations would be necessary to cope with them. The Association for responsible research and innovation in genome editing is precisely requesting this effort. The only precedents are the Declaration of Human Rights and it seems that it will not be an easy task to fulfill.
PS. Check the former post on Nuffield reports on this topic
02 de gener 2020
Fighting against techno-eugenics
A Chinese scientist who shocked the medical community last year when he said he had illegally created the world's first gene-edited babies has been sentenced to three years in prison by a court in southern China.Such unethical medical behavior is the worst news of 2019. And this article explained last June the reasons:
He Jiankui announced in November 2018 that he had used a powerful technique called CRISPR on a human embryo to edit the genes of twin girls. He said he modified a gene with the intention of protecting the girls against HIV, the virus that causes AIDS. Many scientists expressed concerns about possible unintended side effects of the genetic changes that could be passed down to future generations.
Last fall, He also indicated there might be another pregnancy involving a gene-edited embryo. The court indicated that three genetically edited babies have been born.
The closed court in Shenzhen found He and two colleagues guilty of illegal medical practice by knowingly violating the country's regulations and ethical principles with their experiments, Xinhua news agency reported. It also ordered He to pay a fine of about $430,000.
The link between CCR5 and HIV is fairly well studied. Disabling CCR5 removes the doorway HIV uses to enter and infect cells, but it does so only for some strains of HIV; there are others that don’t need CCR5. Further, the genetic sequence He’s edits produced does not match this well-studied variant of CCR5; in fact, it has never been observed in humans or animals. In other words, no one has any idea whether the variant with which Lulu and Nana are now living will affect HIV immunity or anything else.And this article reminds us that, despite the appearance of agreement, ethical questions that have surrounded human germline editing for years have yet to be properly addressed.
That’s a key issue: Genes don’t do just one thing. Most illnesses and traits are influenced by dozens, hundreds, even thousands of DNA variations. Each of our roughly 20,000 genes is linked to many different aspects of our physiology and health. So what else does CCR5 do? A variant that provides protection against HIV also seems to increase susceptibility to a number of more common diseases, like flu and West Nile virus.
CCR5 has also been linked to brain function, which led to some sensational headlines and media speculation that the gene-edited babies might have enhanced brains. There are likely myriad other processes to which CCR5 contributes that we don’t know about yet. To that point: before the recent study, no one had researched whether the CCR5 mutation resulted in better or worse health over a person’s lifetime.
The CCR5 story illustrates a flaw in the logic that underlies gene editing. Efforts to change one gene to affect one illness in a future person ignore the fact that health is the result of infinitely complex interactions within and outside a person’s body. In most cases, the presence or absence of a particular genetic variant is not the sole determinant of a disease or condition.
15 de gener 2021
Precision medicine
Precision Medicine for Investigators, Practitioners and Providers
Many topics under the same umbrella:
Table of Contents
Introduction
2. Role of genomics in precision medicine
3. High throughput omics in the precision medicine ecosystem
4. Infant gut microbiome
5. Paraprebiotics
6. Fecal transplantation in autoimmune disease
7. Drug pharmacomicrobiomics
8. CRISPR technology for genome editing
9. Engineering microbial living therapeutics
10. Organ on a chip
11. Multicellular in-vitro organ systems
12. The role of biobanks in biomarker development
13. Translational interest of immune profiling
14. Organoid pharmacotyping
15. Large datasets for genomic investigation
16. Modern applications of neurogenetics
17. Genomic profiling in cancer
18. Genomics in pediatrics
19. Genomics of gastric cancer
20. Genomics of prostate cancer
21. MicroRNAs and inflammation markers in obesity
22. MiRNA sequencing for myocardial infarction screening
23. Cell free DNA in hepatocellular carcinoma
24. Non coding RNA in cancer
25. Germline variants and childhood cancer
26. Pharmacogenomics in cancer
27. Proteomic biomarkers in vireoretinal disease
28. Proteomics in respiratory diseases
29. Cardiovascular proteomics
30. Host genetics, microbiome, and inflammatory bowel disease
31. Sampling, Analyzing, and Integrating Microbiome ‘omics Data in a Translational Clinical Setting
32. Omics and microbiome in sepsis
33. Molecular and omics methods for invasive candidiasis
34. Lipid metabolism in colorectal cancer
35. Salivary volatolome in breast cancer
36. immunodiagnosis in leprosy
37. decision support systems in breast cancer
38. Electronic medical records and diabetes phenotyping
39. Clinical signature of suicide risk
40. Machine learning and cluster analysis in critical care
41. Artificial intelligence in gastroenterology
42. Algorithms for epileptic seizure prediction
43. Precision medicine in ophthalmology
44. Phenotyping COPD
45. Lifestyle medicine
46. Precision medicine for a healthier world
47. Aging and clustering of functional brain networks
48. Nutrigenetics
49. Genome editing in reproductive medicine
50. MRI guided prostate biopsy
51. Precision Nutrition
52. Theranostics in precision oncology
53. Precision medicine in daily practice
54. Imaging in precision medicine
55. Organoid for drug screening
56. Printing of personalized medication using binder jetting 3D printer
57. 3 D printing in orthopedic trauma
58. Consumer genetic testing tools in depression
59. The future of wearables
60. Tumor heterogeneity and drug development
61. Smartphone based clinical diagnosis
62. Smartphone biosensing for point of care use
63. Data security and patient protection
64. Blockchain solutions for healthcare
65. Ethical questions in gene therapy
66. Pitfalls of organ on a chip technologies
67. Regulatory issues of artificial intelligence in radiology
68. Academic industrial alliance
69. The future of precision medicine
70. Precision Medicine Glossary
71. Useful internet sites