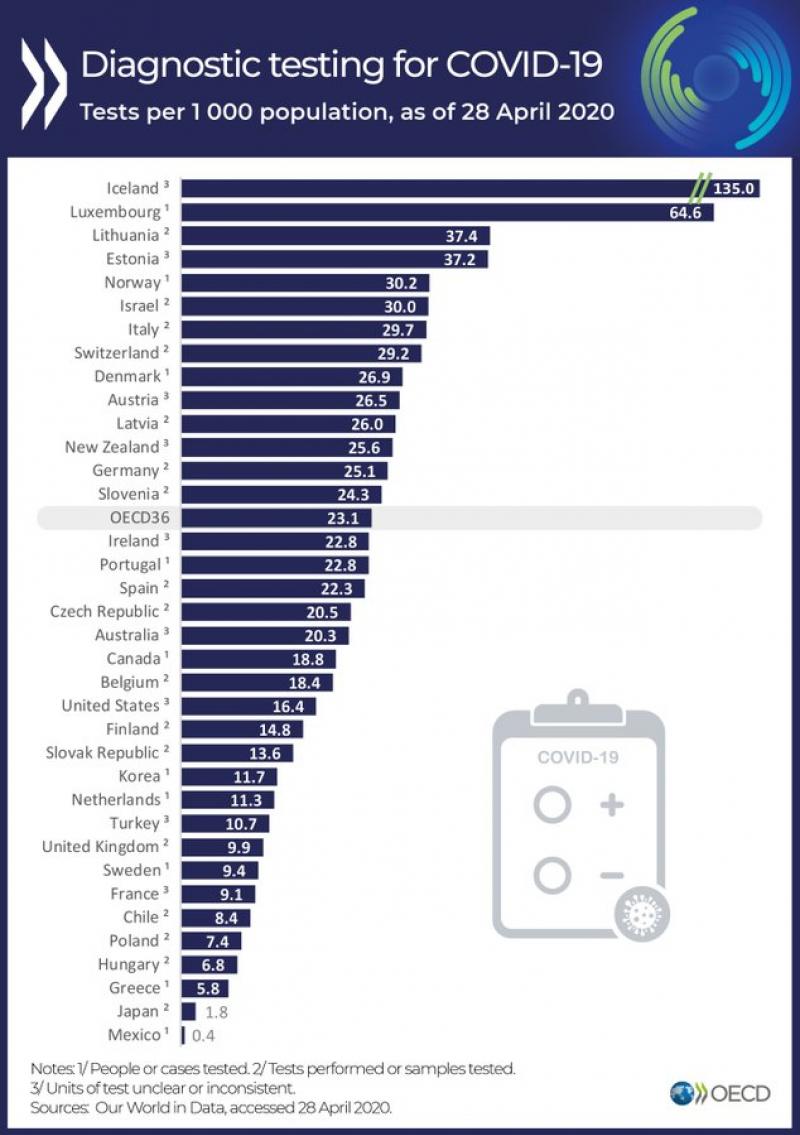
The evidence of market failure during this pandemic is everywhere. Shortages, excessive prices, unavailable capacity...It is a clear example of mismatch between demand and supply. The question is, Can we do it otherwise?. In
this article there are some hints for resource allocation for testing activities.
Globally, the development of diagnostics has long been left to markets, many of which are highly specialized. But while there are diagnostics markets for major infectious and non-infectious diseases, and even neglected tropical diseases, there is none for pandemic diseases.
Governments can of course counteract market deficiencies, but the commonly used mechanisms still require a trace level of demand, which does not exist for pandemic-disease diagnostics until the brink of an outbreak. And national governments, subject as they are to political and ideological constraints, cannot be relied upon always to create markets with the same swiftness demonstrated by South Korea. Reactive market creation is therefore not the way forward.
Instead, national governments should support the creation of a global coordinating platform for pandemic preparedness. Such a platform can take the lead in raising and pooling capital to channel toward rapid development, production, and distribution of diagnostics for pandemic diseases.
The blueprint for such a platform already exists. The Coalition for Epidemic Preparedness Innovations (CEPI) is a coordinating mechanism focused on advancing vaccine development and facilitating clinical validation, mass-scale manufacturing, and stockpiling. By reducing uncertainty and minimizing disruptions, CEPI makes vaccine markets more secure, accessible, and dynamic.
CEPI relies on both traditional financing (large grants from governments and foundations) and innovative financing (the returns from instruments like the International Finance Facility for Immunization, or IFFIm). In the event of an outbreak, CEPI uses instruments like Advanced Market Commitments (AMCs) or volume guarantees – which can be structured through mechanisms like the Global Health Investment Fund and InnovFin, or as conditional pledges to IFFIm and Gavi, the Vaccine Alliance – to enable it to scale up production quickly.
This blueprint can easily be replicated for diagnostics. All that is needed is a specialized entity – an institution or initiative that couples research and development with market access.