Big Pharma. The Money Behind the Pills
Contents:
Chapter 1
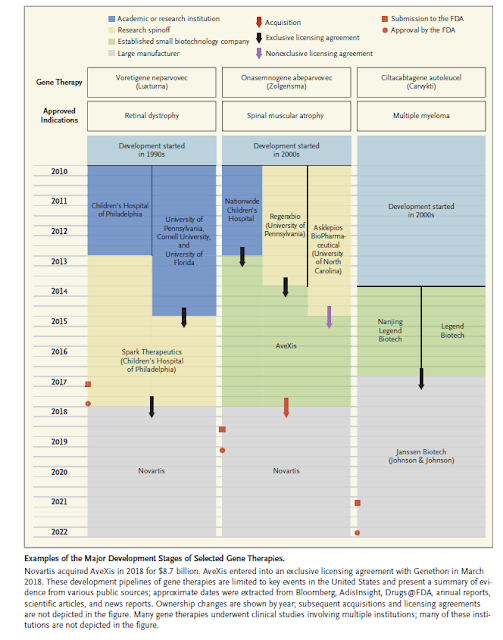
Big Pharma’s New Deal: Acquisition and Little Innovation
Blockbuster Drugs Are So Last Century BY ALEX BERENSON
When Academia Puts Profit Ahead of Wonder BY JANET RAE-DUPREE
Grant System Leads Cancer Researchers to Play It Safe BY GINA KOLATA
Are Doctors Too Wary of Drug Companies? BY PAULINE W. CHEN, M.D.
Valeant’s History of Deal-Making BY WILLIAM ALDEN
Roche to Buy InterMune for $8.3 Billion BY ANDREW POLLACK AND MICHAEL J. DE LA MERCED
Why Are So Few Blockbuster Drugs Invented Today? BY DAN HURLEY
$2.6 Billion to Develop a Drug? New Estimate Makes Questionable Assumptions BY AARON E. CARROLL
Stop Subsidizing Big Pharma BY LLEWELLYN HINKES-JONES
Ways to Fund Research on Rare Diseases THE NEW YORK TIMES
AstraZeneca to Acquire Majority Stake in Acerta Pharma BY CHAD BRAY
Explaining Valeant: The Main Theories BY STEVEN DAVIDOFF SOLOMON
Chapter 2
Monopolies and Exclusivity Drive Price Spikes
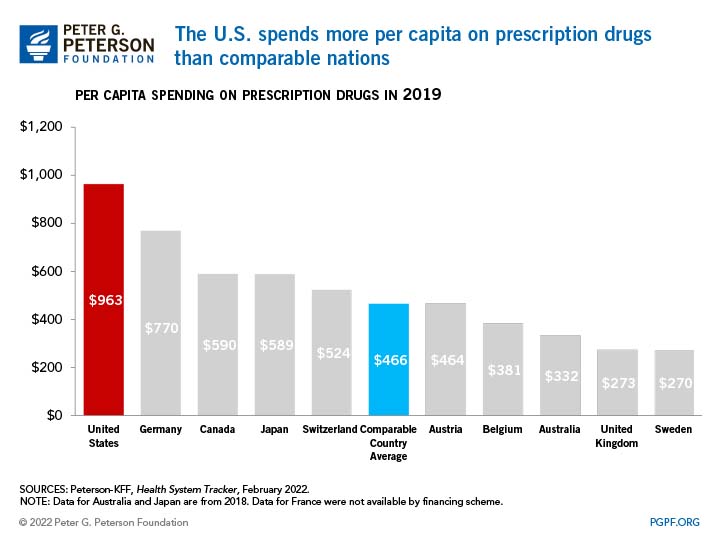
Runaway Drug Prices BY THE NEW YORK TIMES
Costly Hepatitis C Drugs for Everyone? BY THE NEW YORK TIMES
New Cholesterol Drugs Are Vastly Overpriced, Analysis Says BY ANDREW POLLACK
Inflated Drug Prices THE NEW YORK TIMES
Drug Goes From $13.50 a Tablet to $750, Overnight BY ANDREW POLLACK
Big Price Increase for Tuberculosis Drug Is Rescinded BY ANDREW POLLACK
Valeant Under Investigation for Its Drug Pricing Practices BY ANDREW POLLACK
Senators Condemn Big Price Increases for Drugs BY ANDREW POLLACK
No Justification for High Drug Prices BY THE NEW YORK TIMES
Another Drug Pricing Ripoff BY THE NEW YORK TIMES
The EpiPen, a Case Study in Health System Dysfunction BY AARON E. CARROLL
The Complex Math Behind Spiraling Prescription Drug Prices BY KATIE THOMAS
The Lesson of EpiPens: Why Drug Prices Spike, Again and Again BY ELISABETH ROSENTHAL
Chapter 3
Disease Branding and the Profusion of Diagnoses
Ritalin Wars BY JUDITH WARNER
Disease Branding BY BEN SCHOTT
Still the ‘Age of Anxiety.’ Or Is It? BY DANIEL SMITH
Ruling Is Victory for Drug Companies in Promoting Medicine for Other Uses BY KATIE THOMAS
A.D.H.D. Seen in 11% of U.S. Children as Diagnoses Rise BY ALAN SCHWARZ AND SARAH COHEN
Is It Really A.D.H.D. or Just Immaturity? BY KJ DELL’ANTONIA
Overselling A.D.H.D.: A New Book Exposes Big Pharma’s Role BY STEVE SILBERMAN
A Profusion of Diagnoses. That’s Good and Bad. BY DHRUV KHULLAR, M.D.
Chapter 4
The Money Behind Epidemics: Preventing, Treating and Healing
For Profit, Industry Seeks Cancer Drugs BY ANDREW POLLACK
F.D.A. Advisory Panel Backs Preventive Use of H.I.V. Drug BY DENISE GRADY
Advocating Pill, U.S. Signals Shift to Prevent AIDS BY DONALD G. MCNEIL JR.
Painkillers Resist Abuse, but Experts Still Worry BY ALAN SCHWARZ
The C.E.O. of H.I.V. BY CHRISTOPHER GLAZEK
The Insanity of Taxpayer-Funded Addiction BY THE NEW YORK TIMES
F.D.A. to Expand Medication-Assisted Therapy for Opioid Addicts BY SHEILA KAPLAN
As Opioid Prescriptions Fall, Prescriptions for Drugs to Treat Addiction Rise BY ABBY GOODNOUGH
Chapter 5
The Trump Administration vs. Big Pharma
The Real Reason Medicare Is a Lousy Drug Negotiator: It Can’t Say No BY MARGOT SANGER-KATZ
The Fight Trump Faces Over Drug Prices BY KATIE THOMAS
Trump Vows to Ease Rules for Drug Makers, but Again Zeros In on Prices BY KATIE THOMAS
Drug Lobbyists’ Battle Cry Over Prices: Blame the Others BY ERIC LIPTON AND KATIE THOMAS
Draft Order on Drug Prices Proposes Easing Regulations BY SHEILA KAPLAN AND KATIE THOMAS
Lower Drug Prices: New Proposals Carry Lots of Promises BY KATIE THOMAS AND REED ABELSON
What Big Pharma Fears Most: A Trump Alliance With Democrats to Cut Drug Prices BY ROBERT PEAR
Trump Proposes to Lower Drug Prices by Basing Them on Other Countries’ Costs BY ROBERT PEAR