Informe de la BioRegió 2022. El sector de les ciències de la vida i la salut a Catalunya
El dinamisme de la recerca en ciències de la vida a Catalunya és indubtable. Es mou però cap a on?. Avança realment o només crea expectatives?.
He estat aquests dies a BioSpain2023, una trobada clau del sector, on el paper de les empreses catalanes també és notori. Hi havia 800 empreses en total, gairebé 2000 assistents. Però el més important és què es comprava i què es venia.
Hi havia 744 actius per vendre, 323 productes i 1478 serveis. Les xifres impressionen, certament. I alguns poden preguntar-se quins són els actius en venda? Doncs fonamentalment patents i llicències. Per exemple, en miro una d'un institut públic (Sant Pau/UAB) "FANCONINIB – TARGETING DNA REPAIR
IN CANCER THERAPEUTICS" (Fanconinib presents a new mechanism of action for cancer treatment that targets DNA repair and aims to "Fanconize" cancer cells and kill them by breaking their chromosomes through the inhibition of the FA/BRCA pathway. Currently there are no specific drugs in the market with inhibitory effect on this pathway). L'origen és una extensió d'indicació sobre un medicament existent.
I per què se'n diuen actius, si ningú sap com anotar-los com a tals en una comptabilitat? Doncs perquè és l'exemple més clar de financialització dels medicaments. Ho vaig explicar el mes de febrer passat comentant el gran llibre de Victor Roy, "Capitalizing a cure". Allà explica que el que es compra i es ven són expectatives financeres sobre teràpies més o menys elaborades que cotitzaran a borsa .
Miro quan és la inversió de 2022 en startups a Catalunya i em diu 455 milions, i algú treu pit com si fos molt. Miro què ha fet una sola empresa i Pfizer aquest any veig que ha comprat Seegene per 43 mil milions de dòlars, xifra record. Podem dir que Pfizer ha pagat cara l'externalització de la recerca d'un medicament oncològic (Conjugat Anticos-fàrmac ADC)? No ho sabem, ara bé el dia que el posi al mercat recordeu la xifra que li ha costat allunyar-se del risc de la R+D.
Les diferències en les xifres d'inversió entre noves empreses i gran indústria farmacèutica són abismals. Les notícies que es generen a premsa per les primeres són moltes però mai sabem on acaben. Les segones, són poques i acaben en un preu estratosfèric, que llavors si que és notícia.
Algú hauria de posar una mica de seny i realisme a tot plegat. Cal admetre que la musculatura de la traslació de la recerca a clínica és feble i que no tenim recursos per aplicar-hi, malgrat tenir talent, i molt. La funció de producció va més coixa per la K (de capital) en comparació de la L (de labour/talent). I sobretot per l'entorn/ecosistema que es necessita perquè la K i la L reixin. Més K i prou no es la solució.
Sempre recordaré el dia que jo estava a Berkeley i l'Ed Penhoet em va dir: "mira, els Estat Units ja no som líders mundials en cotxes, ni en molts sectors on la Xina ens ha passat pel davant, ara bé en ciències de la vida ens hi mantindrem. La gent pot parlar de recerca en xarxa i descentralitzada, però nosaltres la controlarem, controlarem la xarxa". És a dir el poder econòmic, la financialització es mantindria a les mans del USA. D'això en fa uns quinze anys. Mireu el CV de l'Ed Penhoet si no el coneixieu abans, i veureu el seu nivell.
I després de tot plegat i en relació a Catalunya, m'agradaria veure els resultats de la inversió en recerca pública, on són les patents? què han costat? quants diners n'hem recuperat i qui és l'últim tenidor?. Fa molts anys que ho busco i no ho trobo. Potser perquè no existeix, o potser perquè no interessa que existeixi. Tant costaria un registre d'actius públics? Qüestió de transparència pendent, una vegada més.
PS. Aquest és un tema que no es pot ventilar amb un escrit curt. Per tant serveixin aquests paràgrafs com a punt de reflexió tant sols.
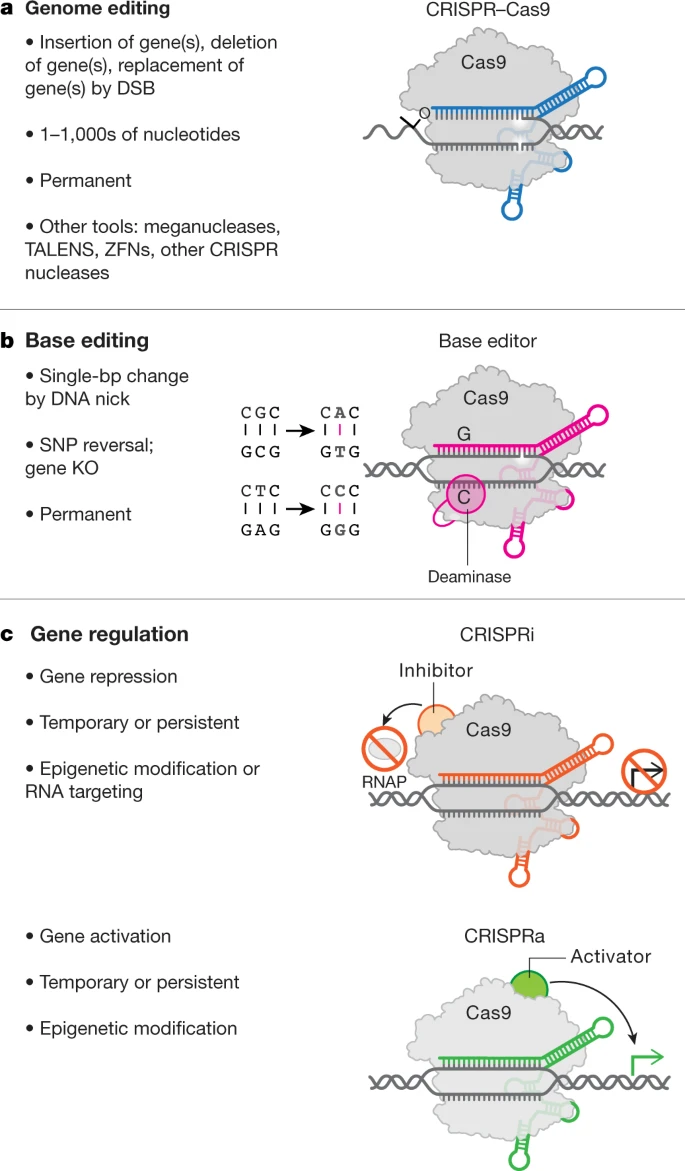
PS. He posat al buscador d'actius CRISPR i em trobo que només n'hi ha 8 (cap provinent de Catalunya). Increïble. Ningú podria imaginar als USA un congrés Biotech on 8 dels 744 actius, un 1% fos CRISPR a data d'avui (0% de Catalunya). No m'he mirat el detall però suposo que són olinucleòtids, allò que es pot patentar.
Rocio Madrid al KBR